Entering into a new market segment is not necessarily an easy task, and the decision to do so must be based on sound realities and facts. In the present article, the author examines the sulfate-free segment and discusses questions that R&D must ask about sulfates, as well as facts about them, to assist in making the choice of whether or not to enter this market segment.
To begin, it may be helpful to consider why consumers are interested in sulfate-free products in the first place. As most formulators know, questionable science often spurs misleading information that produces media hype and confuses consumers. Following is just a sampling of claims that Web sites have made about sulfates, including cautions against using personal cleansers containing sodium/ammonium lauryl/laureth sulfates:
1. Sodium/ammonium lauryl and laureth sulfate are harsh detergents that are used in garage cleaners, car washes and engine degreasers and, as such, they should not be used in personal cleansing products. This claim is obviously not based on sound science; the fact that an ingredient is used in engine degreaser does not mean it should not be used in a personal care product. While sulfates have occasionally been linked to irritation potential, formulas can be designed to safely maximize on their benefits. A wide variety of surfactants and other ingredients are used both in the personal care and detergent industries. For example, cocamidopropyl betaine is used in dishwashing liquids, shampoos and body washes. Betaines can also be used in car wash soaps because of their high-foaming, viscosity-building and foam stabilization properties. Many amphoteric and nonionic surfactants such as amphoacetates and alcohol ethoxylates are used in personal care products as well as industrial and institutional (I&I) cleaners.
2. Sodium lauryl sulfate is an estrogen-mimic and may be responsible for increasing breast cancers. Without a scientific study by a reputable organization and a conclusion drawn to this effect, such unfounded claims do not justify the removal of sulfated surfactants from personal care products.
3. Studies show that use of sodium lauryl sulfate in toothpaste may increase recurrent aphthous ulcers,1 also known as canker sores. Studies also exist concluding that both SLS-free and SLS-containing dentifrices have similar effects.2
In general, those Web sites and Internet blogs that describe the “dangers” of sodium lauryl/laureth sulfate do not back these claims with scientific data, facts and/or studies. Regulatory agencies such as the US Food and Drug Administration (FDA) have deemed sodium lauryl sulfate as safe for use in food.3 Sodium lauryl sulfate and ammonium lauryl sulfate also are approved as “indirect food additives.”
The Cosmetic Ingredient Review (CIR) Expert Panel has evaluated the available scientific data and concludes that sodium lauryl sulfate and ammonium lauryl sulfate are safe in formulations designed for discontinuous, brief use, followed by thorough rinsing from the surface of the skin.4 In 2002, the CIR Expert Panel considered new data on sodium lauryl sulfate and ammonium lauryl sulfate and reaffirmed this conclusion.
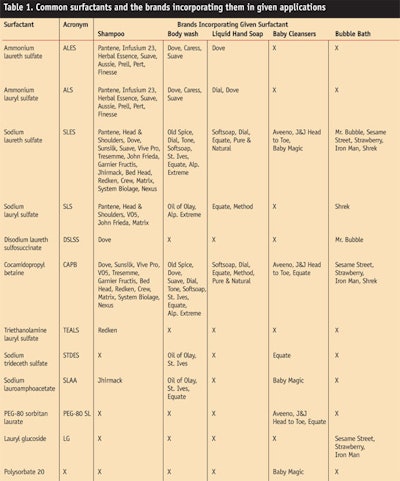
In fact, many cleansers marketed under major brand names, including shampoos, body washes, liquid hand soaps, baby washes and bubble bath (see Table 1), that are currently on the US market, contain lauryl and lauryl ether sulfates as the main or primary surfactant and these products are manufactured by well-reputed, global market leaders. The information shown in Table 1 was collected via a random survey of a local chain store’s shelves by the author.
In addition to unfounded claims against sulfates, which have spurred consumer interest in sulfate-free
products, differences in cost between sulfate-containing and sulfate-free products provide an interesting point of discussion. For example, a 16-oz. bottle of sulfate-containing shampoo may sell for US $0.99 to $5.00 but the same amount of sulfate-free shampoo or body wash, particularly if it includes natural or organic materials, can cost up to $50.00. This higher cost would tend to drive consumer purchases toward sulfate-containing shampoos, which might suggest this “sulfate-free” campaign was invented as a technique to generate sales for higher-priced, sulfate-free products, rather than a scientific reason to remove sulfates.
The demand for sulfate-free products is similar to natural or organic in the sense that these words attract consumers. While this is a separate topic unto itself, it may be worth noting here, as many chemists know, that so long as an ingredient has an identical chemical composition and structure, its effects on human hair and skin will most likely be the same—regardless of being natural or synthetic. Currently, there is no 100% natural substance that works as effectively as typical surfactants but surfactants can be derived from natural feedstocks such as coconut and palm oils. In fact, many surfactant suppliers in North America use these natural, vegetable, renewable feedstocks to make sodium/ammonium lauryl sulfates; these surfactants are also biodegradable.
Cost vs. Benefits
One important aspect of product development and marketing involves cost/performance optimization. In other words, to be competitively priced, a product should be developed at minimum cost while also maximizing on its benefits.
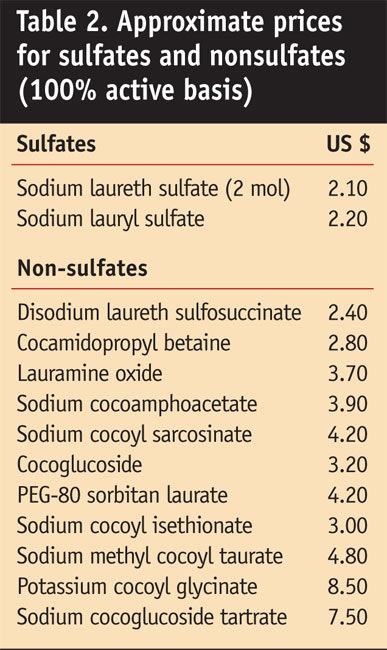
Cost: General market prices of various surfactants are shown in
Table 2. It is important to note that these prices are estimates and that many are based on company list prices; in addition, note that prices tend to fluctuate, especially in recent months. Overall, sulfated surfactants are less expensive than sulfate-free surfactants on a per-pound active basis. A comparison of costs for nonsulfate- vs. sulfate-containing formulas is shown in Formulas 1a and 1b.
Sulfate-free surfactants generally cost more than lauryl/laureth sulfates because of higher feedstock and processing costs. As will be shown, many sulfate-free surfactants also do not produce as effective lather profiles as sulfates and thus require higher surfactant loadings in sulfate-free formulas. Another factor for the higher cost of sulfate-free formulas is their thickening or viscosity building costs. As will be described, formulas based on sulfated surfactants can be thickened with relatively inexpensive materials such as salt and alkanolamides; sulfate-free formulas may require more expensive thickeners such as polymers or highly ethoxylated esters.
Benefits
Regardless of cost, in personal cleansers such as shampoos and body washes, there are two primary physical properties that are near and dear to the hearts of consumers—foam or more accurately, lather, as well as viscosity. The lather profile of a shampoo will include traits such as quantity of overall foam, quantity and quality of flash foam, creaminess of lather, the time it takes to generate a good lather on hair, wetting and spreading of lather over the hair, cleaning performance of the lather, rinseability, residue left after shampooing, and the afterfeel together with the manageability of the hair including wet combing and dry combing aspects. In regard to viscosity, shampoos should appear thick and viscous—runny, watery products are unappealing to consumers and are thus not marketable.
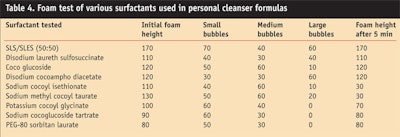
The foam heights and viscosities of the two formulas in Formula 1a and 1b were compared. A cylinder shake foam test was performed to measure foam height, and the viscosity was measured at 25˚C using a viscometera, spindle #6, at a speed of 10 rpm. The results of these tests are summarized in Table 3.
Foam/lather test: First, 10% active surfactant, 2% cocamide DEA, and 3% cocamidopropyl betaine were combined with water to 100% and mixed well to obtain a clear, smooth and homogenous mixture. Then,
250 mL of the test solution was measured in a plastic graduated cylinder and quickly inverted into a 1000-mL glass cylinder and removed.
The foam height was read immediately and the amounts of small, creamy bubbles, medium-sized bubbles and large bubbles were recorded—smaller bubbles produce creamy lather whereas larger bubbles provide good flash foaming effects. Finally, the foam volume was read again after 5 min to determine the foam’s stability. The lesser the difference between the immediate foam height and the foam height after 5 min, the more stable the foam.
As shown in Table 4, the mixture of sulfated surfactants, namely sodium lauryl sulfate and sodium laureth (2 mol EO) sulfate, produced the highest quantity of foam. This mixture also produced the highest amount of small bubbles and had good foam stability for 5 min. This suggests that a shampoo incorporating these sulfated materials would have efficacious foaming properties, both quantitatively and qualitatively.
The three surfactants at the bottom of the table, potassium cocoyl glycinate, sodium cocoglucoside tartrate and PEG-80 sorbitan laurate, produced the least amounts of foam. Sodium cocoyl isethionate and sodium methyl cocoyl taurate produced the most unstable foam among the surfactants tested. Finally, disodium cocoamphodiacetate produced the highest amount of relatively larger bubbles. Overall, sulfates had either equal or better foam stability than sulfate-free surfactants.
Viscosity: As noted, viscosity is another important factor in shampoo formulations. A formula based on 10% active 50:50 mixture of sodium lauryl sulfate and sodium laureth sulfate would only need 1% of an alkanolamide and 2–4% salt to bring the viscosity to approximately 5000–10,000 cp, which is generally accepted as a good, marketable viscosity for shampoo products. This cost would roughly be about 1.5 cents per pound of the formula. However, when a shampoo is formulated with nonsulfated surfactants, the per pound cost of thickening the formula rises because many nonsulfated surfactants require either higher amounts of alkanolamides, combinations of alkanolamides and additional secondary surfactants such as alkylamidopropyl betaine, or nonalkanolamide thickeners; these all are typically more expensive. Thus, sulfated surfactants require less expensive thickeners (or less thickener of the same quality) to build the viscosity, compared with sulfate-free surfactants.
Discussion and Conclusion
In the present article, sulfate vs. sulfate-free formulating is discussed to assist cosmetic chemists in making formulation choices. Sulfate surfactants such as sodium and ammonium lauryl sulfates, and sodium and ammonium laureth sulfates, have been found safe for use in wash-off cleansing products by the CIR Expert Panel.
Sulfated surfactants also are less expensive when compared with the majority of sulfate-free surfactants. They also provide higher viscosity and foam height when used in shampoos, body washes, liquid hand soaps and baby washes. In addition, sodium and ammonium lauryl sulfates can be derived from coconut oil—a natural, renewable and vegetable feedstock—thus they can be marketed as “naturally derived” and they are biodegradable, fitting eco-friendly claims.
Many well-known mass-marketed and professional salon brands of personal cleansing products use sulfated surfactants in their products, including baby cleansers. And while some sulfate-free surfactants do bring desired properties to formulas such as mildness, experienced formulators can develop formulas that combine the excellent lather profile of sulfates with the mildness of nonsulfated surfactants. Thus, a combination of sodium laureth sulfate, disodium laureth sulfosuccinate and cocamidopropyl betaine in equal-active ratio can provide a surfactant base that has an excellent lather profile with the mildness of the sulfosuccinate and betaine. Ethoxylated sorbitan esters can be added to further cut down the irritation potential of a formula, and skin and hair conditioners such as silicones, cationic polymers and/or fatty esters can be incorporated to overcome the drying effects of some sulfated surfactants.
In the end, the cost/performance advantages of sulfated surfactants are the main reason that many major manufacturers continue to use sulfates as primary surfactants in their products. Furthermore, little sound science exists to support the current “sulfate-free” market trend, especially when considering the potential benefits sulfates can impart in formulations and provide to formulators.
Disclaimer: The information contained herein is provided in good faith as starting guideline to personal care product formulators, and is based on studies conducted in the author’s laboratories and on the work of others. Pilot Chemical Company makes no warranties, expressed or implied, as to the accuracy of the information contained herein. Nothing contained herein grants or extends a license or permission in connection with patents of Pilot Chemical Co. or others.
References
1. L Chahine, N Sempson and C Wagoner, The effect of sodium lauryl sulfate on recurrent aphthous ulcers: A clinical study, Compend Contin Educ Dent 18(12)1238–40 (Dec 1997)
2. CM Healy, M Paterson, S Joyston-Bechal, DM Williams and MH Thornhill, The effect of a sodium lauryl sulfate-free dentifrice on patients with recurrent oral ulceration. Oral Diseases 5(1) 39–43 (Jan 1999)
3. US Code of Federal Regulations, [21 CFR 172.822 (4/1/99)], [21 CFR 175.300 (4/1/99)], [21 CFR 175.320 (4/1/99)], available at www.accessdata.fda.gov/SCRIPTs/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=172 (Apr 1, 2008)
4. CIR Reference Table (includes a complete list of all findings), Cosmetic Ingredient Review Web site, available at: www.cir-safety.org/staff_files/ReferenceTable.pdf (Accessed Dec 29, 2008)