Read the full article in the January 2021 digital edition. . .
Acknowledgments: The authors would like to acknowledge the services of Augustine Scientific for surface tensiometry measurements, and thank Chris Rulison, Ph.D. of Augustine Scientific for his helpful insights and thoughtful discussions of the surface tensiometry results. The authors also acknowledge the services of the Institute for In Vitro Sciences for conducting in vitro studies of irritation potential.
Surfactants remain among mankind’s most potent tools for preventing the spread of infectious disease and facilitating well-being via improved personal hygiene. The COVID-19 global pandemic has underscored this fact, as illustrated by public service announcements promoting frequent hand-washing and the increased consumer demand for surfactant-based cleansers during this period, including liquid hand soaps, body washes, etc.1
Nevertheless, the decades-old dilemma of surfactant-induced skin irritation remains ever-present2 ,3 and risks to skin barrier health are now greater due to the increase in chronic surfactant exposure resulting from the greater frequency of personal cleansing, coupled with greater use of hand sanitizers and exposure to disinfecting products for hard surfaces. Past studies of health care workers have demonstrated the negative consequences of repeated surfactant exposure on the skin, particularly on the hands, and provide insight into effects that may now extend to the general population.4, 5
The damage to the skin barrier from harsh surfactants can manifest itself as everything from the aesthetically unappealing “dry skin,” to the more severe irritant contact dermatitis with clinical symptoms including redness, swelling and itching. Skin barrier damage also increases susceptibility to allergic contact dermatitis, as the compromised skin barrier is more permeable to allergenic compounds.6 Thus, the potentially harmful side effects of surfactants on skin health must be managed accordingly so that consumers can realize the profound health benefits of these ingredients without negative consequences.
Formulators face the difficult challenge of creating cleansing products that:
- Are effective at removing dirt and germs yet also mild to the skin;
- Provide consumer-preferred attributes, such as easy lathering, to produce copious amounts of foam; and
- Are economical, to remain competitive in a highly commoditized category.
Thus, the goal remains: to formulate high-foaming, harsher commodity surfactants into high performance cleansers that are mild to skin. This paper will demonstrate the utility of polyesteramine conditioning polymers as a simple solution for increasing the mildness of surfactant-based cleansers for improved skin barrier health. By taking advantage of polyesteramines’ strong interactions with surfactants, formulators can use these surface-active polymers to reduce the irritation potential of traditionally harsh surfactants without compromising clarity or foaming behavior, and while simultaneously obtaining the benefits of conditioning and viscosity enhancement in cleanser formulations.
Irritation Mitigation
The classic approach to formulating gentler surfactant-based cleansers involves the selection of milder surfactants, e.g., sodium cocoyl isethionate instead of sodium lauryl sulfate; and the formulation of multicomponent surfactant blends designed to lower irritation potential, e.g., via the combination of anionic surfactants with milder zwitterionic and nonionic varieties.7-9 Although these formulation strategies provide milder cleansers, they are often accompanied by drawbacks such as compromised foaming performance, which is a critical element of the consumer experience, and significantly greater raw material costs. More recently, methods based on polymer technologies have proven successful for overcoming the challenge of surfactant-induced irritation—most notably, polymer-surfactant association with hydrophobically modified polymers (HMPs)10 and the use of polymeric surfactants comprising multiple amphiphilic repeat units.11
Walters, et al., introduced the concept of utilizing low molecular weight HMPs to reduce surfactant irritation potential without negatively impacting other aspects of the cleansing formulation such as clarity, rheological profile or foaming behavior.12, 13 The adsorption of surfactants onto HMPs to form HMP-surfactant complexes was demonstrated and quantified using equilibrium surface tensiometry and helped to articulate the mechanism of irritation mitigation.
Surfactants in aqueous solutions normally exist as self-assembled free micelles in equilibrium with a small concentration of free monomeric surfactant; however, in the presence of HMPs, a significant fraction of the surfactant species binds to the HMP, effectively reducing the concentration of free surfactant that is available to interact with skin. In other words, HMPs provide an additional sacrificial substrate other than skin for surfactant binding. This surfactant-binding mechanism was shown to be highly effective at reducing the ability of surfactant-based cleansers to penetrate and disrupt the skin barrier.14, 15 Nevertheless, the HMP-surfactant complexes are dynamic, surface-active species and can still participate in phenomena such as the solubilization of hydrophobic compounds and stabilization of air-water interfaces (i.e., foaming). As such, HMPs can be deployed to improve cleanser mildness without compromising formulation properties or performance.
Polyesteramines
Polyesteramines are polymers comprised of repeating units linked by ester bonds (polyesters) that also bear amine functionalities, typically in the form of an amine group located in the polymer backbone. Polyester-11 (PE-11)a and Polyester-37 (PE-37)b are branched polyesteramines synthesized by the condensation polymerization of adipic acid, bis-(hydroxyethyl)methylamine, glycerin and either coconut fatty acid (PE-11) or isostearic acid (PE-37), producing only water as a byproduct.16-19 Figure 1 shows the generalized chemical structure of PE-11 and PE-37.
The polymer backbone is comprised primarily of diethylmethylamino adipate repeat units, which incorporate tertiary amine functionalities in the polymer chain. The trifunctional glycerin monomer results in glyceryl adipate branch units in the polyesteramine chain and the monofunctional fatty acids are incorporated as endcaps on the polymer chains. It should be noted that due to the branched nature of PE-11 and PE-37, there will be multiple fatty acyl endcaps on each polymer chain. PE-11 and PE-37 are relatively low molecular weight, polydisperse polymers, with average molecular weight values ranging from 2,700-5,800 g/mol. The polyester backbones are hydrolytically stable under normal shelf-life and accelerated stability conditions, yet readily cleaved by enzymatic action, thus rendering the polyesteramines biodegradable in the environment.
Although PE-11 and PE-37 are polar polymers due to their ester and amine functional groups, they are hydrophobic in nature and only dispersible in water when the amine groups are protonated to render the polymers cationic (see Figure 2). Protonation occurs when the polyesteramine is dispersed in aqueous media and the solution pH is decreased by the addition of acid. Optimal water solubility occurs below pH 6.0 and is dramatically improved in the presence of surfactants.
Unlike permanently charged quaternary ammonium polymers, e.g., polyquaterniums, polyesteramines are pH-responsive and bear transient cationic charges that depend upon the pH of their local environment. PE-11 and PE-37 are rendered amphiphilic in acidic solutions due to the presence of hydrophilic groups (cationic protonated tertiary amines) and lipophilic/hydrophobic groups (the fatty acyl chain ends) within the same macromolecule. This amphiphilic character also renders PE-11 and PE-37 surface-active with the protonated polymers demonstrating a strong affinity for air-water interfaces.
PE-11, PE-37, and related polyesteramines were originally developed in the early 2000s as conditioning polymers for hair and skin care.16, 17, 20 Their combination of cationic and hydrophobic characteristics make PE-11 and PE-37 especially useful as substantive conditioning agents in shampoos and conditioners. However, these same properties also make PE-11 and PE-37 attractive as HMPs for mitigation of surfactant skin irritation. The polymers are well-configured to bind surfactants due to their combination of electrostatic and hydrophobic association mechanisms.21 For electrostatic association, the cationic moieties provide sites for anionic surfactants to bind via ion pairing and in turn, additional surfactants aggregate with the tail groups of the bound anionic surfactants via hydrophobic association. For hydrophobic association, the hydrophobic acyl end groups of the polyesteramines associate with the hydrophobic tail groups of all surfactants, independent of head group type, to participate in the formation of mixed micelles.
Putting these structural properties of polyesteramines to the test, experiments were carried out in test formulations, as described next.
Materials and Methods
Polyesteramines PE-11 and PE-37 were utilized in simple surfactant mixtures intended for use as liquid hand soaps, facial cleansers or body washes. These mixtures would typically need to be combined with specialty surfactants to reduce their irritation potential. The surfactant bases comprised 10% w/w active surfactant at a 3:1 ratio of anionic to a zwitterionic surfactant, with 0.5% w/w sodium benzoate as a preservative. These were adjusted to a pH of 4.7 ± 0.2 using aqueous citric acid. The anionic/zwitterionic surfactant combinations used were sodium C14-16 olefin sulfonate/cocamidopropyl betaine (AOS/CAPB); sodium laureth sulfate/cocamidopropyl betaine (SLES/CAPB); and sodium methyl cocoyl taurate/cocamidopropyl hydroxysultaine (SMCT/CAPHS).
Polyquaternium-7 (PQ-7), a copolymer of acrylamide and diallyldimethylammonium chloride, is a traditional cationic conditioning polymer typically found in hand soaps and body washes.22 It was additionally used in this study for comparisons of polymer-surfactant association and irritation mitigation performance versus polyesteramine conditioning polymers. Its recommended starting use concentration is 1%. 23 For viscosity-building experiments, PEG-120 methyl glucose dioleate was employed as a micellar thickener.24 Formula 1 provides an example of the types of formulations prepared for this study.
Surfactant Binding
Researchers at Johnson & Johnson previously demonstrated that equilibrium surface tensiometry is a highly effective method for quantifying the surfactant-binding capacity of HMPs, and that capacity is directly correlated to a reduction in irritation potential 10, 12, 25 The same method was employed in the present study.
Figure 3 shows the surface tensiometry plot for the AOS/CAPB surfactant system measured at a pH of 4.5, both alone and in the presence of varying levels of PE-11. It should be noted that the concentrations of polymer utilized in the surface tensiometry experiments were on the same order of magnitude as would be encountered during the in-use dilution of a rinse-off cleanser. In the absence of PE-11, the AOS/CAPB surfactant system demonstrated typical surface tension reduction behavior starting at 72 mN/m for pure water (not shown) and decreasing with increasing surfactant concentration until reaching the critical micelle concentration (CMC) value at ca. 0.004% w/w active surfactant and ultimate surface tension of 25.5 mN/m.
By contrast, the solutions of PE-11 exhibited starting surface tension values of 36.8-36.5 mN/m, decreasing slightly with increasing starting polymer concentration. These initial surface tension values at low surfactant concentrations demonstrate the inherent surface activity of the amphiphilic PE-11 molecule in an aqueous solution. As AOS/CAPB is added to solutions of PE-11, the surface tension remains relatively constant and does not begin to decrease significantly until reaching AOS/CAPB concentrations that are one to two orders of magnitude greater than the CMC of AOS/CAPB.
This observation confirms the binding of AOS/CAPB to PE-11 in solution, as the AOS/CAPB is not adsorbing at the air-water interface to lower the surface tension. Rather, it is sequestered in solution as a polymer-surfactant complex. Only at concentrations much greater than the CMC of AOS/CAPB does surfactant binding at the air-water interface become competitive with the adsorption of the surfactant to the polymer. At these concentrations, there is a further reduction of surface tension until it ultimately reaches a minimum at CMCP, the critical micelle concentration of the surfactant mixture in the presence of the polymer.
Figure 4 shows surface tensiometry plots for the AOS/CAPB mixture with 0.2% w/w of either PE-11, PE-37 or PQ-7; the plot of AOS/CAPB in the absence of polymer is shown as well. PE-37 demonstrates slightly greater surface activity compared with PE-11, as indicated by the lower initial surface tension value (35.2 mN/m vs. 36.7 mN/m). This increased surface activity is attributed to the more hydrophobic isostearoyl (branched C18) end groups of the PE-37 versus the cocoyl (C8-C18 linear) end groups found on PE-11. PE-37 also exhibits a slightly greater value of CMCp compared with PE-11. This is also attributed to greater polymer hydrophobicity.
Compared with the amphiphilic polyesteramines, the hydrophilic PQ-7 does not exhibit surface activity and shows a much lower surfactant-binding capacity. The initial surface tension values at low concentrations of AOS/CAPB surfactant mix are identical both in the presence and absence of 0.2% w/w PQ-7, indicating that the polymer is not surface active. The CMCP value for AOS/CAPB in the presence of PQ-7 is dramatically lower compared to the two polyesteramines, indicating that PQ-7 binds much less surfactant.
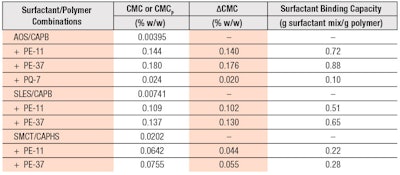
The surfactant-binding capacities of the polymers are quantified by the ΔCMC value, which is the difference between the CMC values in the presence and absence of the polymers, i.e., ΔCMC = CMCP− CMC, and are expressed as ratios of mass of bound surfactant to mass of polymer, i.e., grams of bound surfactant mixture/gram of polymer. Table 1 lists the values of ΔCMC and surfactant-binding capacities for PE-11 and PE-37 with the different surfactant systems used in this study. The surfactant-binding capacity ratios were determined to be independent of polymer concentration within the range of polymer concentrations studied; only the values determined at 0.2% w/w polymer are shown in Table 1.
Viscosity-building and Thickening
Due to their amphiphilic character and propensity to associate with surfactants to form self-assembled aggregates, polyesteramines are expected to influence the microstructure and rheology of surfactant solutions. From a product formulation perspective, additives that increase the viscosity of surfactant mixtures are usually quite desirable, as these additives contribute to product thickening and can reduce the need for additional rheology modifiers. To assess the viscosity-building effects of polyesteramines in the surfactant mixtures of the present study, formulations were prepared with varying levels (0-3% w/w) of PE-11 or PE-37 and then thickened via the addition of a common micellar thickener, PEG-120 methyl glucose dioleate, at 0-2% w/w.
Figure 5a-b shows formulation viscosity as a function of increasing PEG-120 methyl glucose dioleate concentration for the AOS/CAPB surfactant mixtures with either PE-11 or PE-37, respectively. PE-11 built formulation viscosity in the absence of added thickener, reaching a peak viscosity of 1800 cP at 2% PE-11. PE-37 also contributed some viscosity build in the AOS/CAPB system without thickener, although the effect was less pronounced. Viscosities increased dramatically when either PE-11 or PE-37 were combined with PEG-120 methyl glucose dioleate in the AOS/CAPB system, indicating synergistic viscosity building. Maximum synergy occurred at 2% polyesteramine for AOS/ CAPB.
PEG-120 methyl glucose dioleate is reported to build viscosity by increasing micellar size and bridging micelles.26 The observed synergy is attributed to the enhancement of these mechanisms by the polyesteramines, which associate with surfactant micelles via electrostatic and hydrophobic interactions. However, at polyesteramine levels greater than 2%, polymer-surfactant complex formation tends to “consume” surfactant from micelles and becomes competitive with micelle formation, resulting in viscosity decreases as polyesteramine concentration is further increased.
. . .Read more in the January 2021 digital edition. . .
References
- Mintel Group Ltd. (2020). UK hand, body and footcare: Incl impact of COVID-19 market report.
- Rieger, M.M. (19965). Surfactant interactions with skin. Cosmet & Toilet 110(4) 31-50.
- Polefka, T.G. (1999). Surfactant interactions with skin. Handbook of Detergents, Part A: Properties. Marcel Dekker, Inc., New York 443-468.
- Visscher, M.O. and Wickett, R.R. (Oct 2012). Hand hygiene compliance and irritant dermatitis: a juxtaposition of healthcare issues. Int J Cosmet Sci 34(5) 402-415. Available at: https://pubmed.ncbi.nlm.nih.gov/22691060/
- World Health Organization. (2009). Skin reactions related to hand hygiene. WHO Guidelines on Hand Hygiene in Health Care: A Summary 61-63.
- Cork, M. J., et al. (Jul 2006). New perspectives on epidermal barrier dysfunction in atopic dermatitis: Gene-environment interactions. J Allergy Clin Immunol 118(1) 3-21. Available at: https://pubmed.ncbi.nlm.nih.gov/16815133/
- Walters, R. M., et al. (2008). Designing cleansers for the unique needs of baby skin. Cosmet & Toilet 123(12) 53-60.
- Ananthapadmanabhan, K. P., et al. (Jun 2009). A novel technology in mild and moisturizing cleansing liquids. Cosmet Derma 22(6) 307-316.
- Walters, R. M., Gould, R. and Nikolovski, J. (Jun 1, 2020). A delicate dance: mildness and efficacy to cleanse compromised skin. Cosmet & Toilet 135(6) 56-DM36. Available at: https://cosmeticsandtoiletries.texterity.com/cosmeticsandtoiletries/june_2020/MobilePagedReplica.action?pm=2&folio=56#pg89
- Fevola, M.J., LiBrizzi, J.J. and Walters, R.M. (2010). A new approach to formulating mild cleansers: hydrophobically modified polymers for irritation mitigation. Polymeric Delivery of Therapeutics. American Chemical Society, Washington D.C. 221-242.
- Fevola, M. J., et al. (2013). Next generation mildness for personal care: Nonpenetrating polymerized surfactants for cleansing applications. Polymers for Personal Care and Cosmetics. American Chemical Society, Washington D.C. 105-123.
- Walters, R. M., Fevola, M. J. and LiBrizzi, J. J. (Jul 13, 2010). Low-irritation compositions and methods of making the same. US 7,754,666 B2 US. Assigned to Johnson & Johnson Consumer Companies Inc.
- LiBrizzi, J.J., et al. (Sep 28, 2010). Low-irritation compositions and methods of making the same. US 7,803,403 B2 US. Assigned to Johnson & Johnson Consumer
Companies Inc. - Walters, R.M., et al. (Aug 13, 2012). Cleansing formulations that respect skin barrier integrity. Skin Barrier Protection 2012. Available at: https://www.hindawi.com/journals/drp/2012/495917/
- Hornby, S., et al. (May 2016). Effect of commercial cleansers on skin barrier permeability. Skin Res & Tech 22(2) 196-202. Available at: https://pubmed.ncbi.nlm.nih.gov/26094702/
- Burgo, R.B., Smith, D.L. and Ching, H.P. (Mar 24, 2005). Tertiary amine functional complex polyester polymers and methods of production and use. US 2005/0063938 A1 US. Assigned to INOLEX Investment Corp.
- Tang, D. (Sep 5, 2006). Polyesteramine ingredient and use of same. US 7,101,538 US. Assigned to INOLEX Investment Corp.
- On-line INFOBASE ingredient database. (2020). Polyester-11. Monograph ID 22222. Personal Care Products Council. Available at: https://online.personalcarecouncil.org/jsp/IngredientDetail.jsp?monoid=22222
- On-line INFOBASE ingredient database. (2020). Polyester-37. Monograph ID 32935. Personal Care Products Council. Available at: https://online.personalcarecouncil.org/jsp/IngredientDetail.jsp?monoid=32935
- Heldermann, M. and Burch, K. (Sep 14, 2012). Two new options for hair care-optimized polyesteramine technology and the next generation of amidoamine conditioning. SOFW Journal 138(9) 40-42, 44-45. Available at: https://jglobal.jst.go.jp/en/detail?JGLOBAL_ID=201202237604969414
- Goddard, E. D. (1999). Polymer/Surfactant interaction: manifestations, methods, mechanisms. Principles of Polymer Science and Technology in Cosmetics and Personal Care 4. Marcel Dekker, Inc., New York 113-180.
- Fevola, M.J. (2011). Ingredient Profile—Polyquaternium-7. Cosmet & Toilet 126(4). Available at : https://www.cosmeticsandtoiletries.com/formulating/function/moisturizer/premium-Profile-of-Polyquaternium-7-211556881.html
- Lubrizol Advanced Materials, Inc. (2012). Merquat 7SPR polymer technical data sheet.
- Lubrizol Advanced Materials, Inc. Glucamate DOE-120 thickener datasheet.
- LiBrizzi, J.J., et al. (Jan 2, 2007) Methods of reducing irritation in personal care compositions. US 7,157,414 US. Assigned to Johnson & Johnson Consumer Companies Inc.
- Kortemeier, U., et al. (2010). Thickening agents for surfactant systems. SOFW Journal 136 30-38. Available at: https://personal-care.evonik.com/product/personal-care/downloads/public/sofw-thickening-agents.pdf
a Kerazyne MB and b Clarisilk are products of INOLEX, Inc.