Adequate lather is an important factor to consumers when they select toiletries. Another preference is long-lasting and weak acidity (pH 4.5–6.0) on the skin and scalp, which maintains healthy normal skin conditions while suppressing the growth of undesirable microbes and skin irritation. These preferences are met by a noticeable trend in product development to formulate silicones, higher alcohols and a variety of functional materials into toiletries. However, such ingredients, along with primary surfactants, generally suppress lather, and their use can result in slow foaming or reduced volume of foam.1 Thus, there are strong demands from the industry for an ingredient such as an adjuvant surfactant that is compatible with the complicated and diverse formulae necessary to meet consumer demands for both long-lasting, weak acidity and lather. The author describes efforts to develop such a surfactant here.
Increasing Foam
Foaming is important to accelerate the cleaning power of products. While surfactants are used to blend oil-soluble materials with water to carry oils away, the act of foaming removes dirt. This combined effort is typically accomplished with surfactants and/or the addition of other adjuvants, e.g., surfactants having foam-boosting properties. Fatty acid alkanolamides (FAAs) commonly are used in cleaning products and are reported to increase foam volume based on a stabilization effect. Yet, in a preliminary in-house examination, all FAAs failed to significantly increase lathering rate, according to sensory evaluations.
In relation, lathering/foaming and combinatorial studies1 of the physical surface properties of surfactants in a binary system—i.e., primary plus adjuvant surfactants, have reported that surfactants can be incorporated into products so as to increase foam volume. However, no such report has been published for personal care, household or even experimental products regarding lathering rate. Therefore, the focus of the present work was to identify surfactants that could potentially increase lathering rate. Researchers screened many candidates, including all known surfactants, having different polarities, and all that were available both commercially and by in-house synthesis.
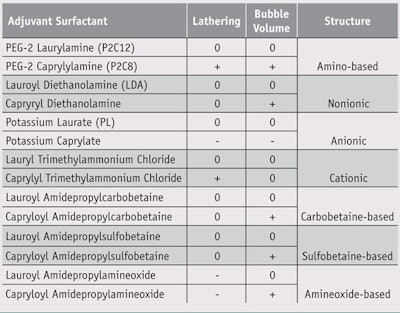
Initial candidates were selected based on: preliminary in-house examinations using an internally developed sensory evaluation method, described later; available data regarding each material’s surface tension; the existing knowledge base; and commercial and practical values—e.g., popularity and availability. Surfactants from a range of classes including amino acid, anionic, cationic, nonionic, carbobetaine, sulfobetaine and aminoxide were then chosen for the sensory evaluation. Interestingly, researchers noted that adding a small amount of PEG-2 caprylylamine (P2C8) (see Figure 1) to primary surfactants having inferior initial bubbling dramatically improved the lathering and stability of the foams produced during the sensory tests.
Despite attempts using various known methodologies to generate foam on products during cleaning, the researchers found that regardless of molecular structure, foams adsorbed to the air-water interface. For example, when a lathering model was simulated by bubbling air into a surfactant aqueous solution via a capillary tube (see Figure 2), the following sequence for lathering occurred.
Step 1: Surfactant molecules quickly adsorbed to the air-water interface in the aqueous solution.
Step 2: The saturation and adsorption of surfactant molecules occurred at the newly formed air-water interface.
Step 3: Lather took place in the states where the two molecular films held water.
Step 4: Other surfactant molecules compensated for the portions of molecules taken away from the system by adsorption. Thus, the critical factors identified for the generation of lather were: surfactant concentration, critical micelle concentration (CMC), free monomers in the aqueous solution, and the surfactant adsorption rate to the air-water interface. The tests conducted are described here in detail.
Materials and Methods
Sensory evaluation: The Ross-Miles method (RMM) is generally employed to study foam stability, and it is known as a simple and informative method to understand the foaming mechanism of surfactants. In this case, the stability of foam films at the air-water interface depends on the existing state of the adsorbed surfactant, the decrease of the drainage rate, and the evaporation rate of water.2 In contrast, the quantitative evaluation of lathering rate (speed) has been difficult because foaming takes place in an extremely short time period of around 30 sec, under a nonequilibrium state, and a specific correlation between the mechanically measurable lathering rate and actual sensory evaluation is not easily observed. Since no specific method was apparent to evaluate lathering between 100 and 1,000 msec, i.e., the initial stage of lathering, a sensory evaluation procedure was developed to assess lathering speed.
The standard formula used for sensory evaluation tests (see Formula 1) was a binary system comprised of an amino acid-type surfactant, sodium lauroyl methylaminopropionate (SLMAP)a as the primary surfactant, and one of the surfactants listed in Table 1 as an adjuvant surfactant. Five trained panelists applied 1 mL of each sample on their hands and evaluated the lathering rate and foam volume by washing their hands for an elapsed time of 30 sec. The panelists judged each sample based on lathering rate and foam volume in comparison with lauroyl diethanolamine (LDA)b as a benchmark, scored as zero. If a sample was judged better than LDA, the score was “+” (positive) and if worse, the score was “–” (negative). Given this short test period, any more complex scoring was considered impractical. Each panelist repeated the test, and the scores given by all panelists were averaged.
Dynamic surface tension: Following sensory evaluations, a comparative, quantitative and correlated study between sensory evaluations and quantitative measurements of the surfactant solutions was conducted. Dynamic surface tension3 (DST), generally used to estimate the physiochemical level of adsorption of a surfactant molecule to the air-water interface, was employed for the quantitative study of the lathering rate. This quantitative measurement was expected to be more reliable and consistent in evaluating and screening the agents that might have a higher lathering rate, and also to reveal any correlation between the quantitative and sensory methods.
Potassium laurate (PL)c was used as the primary surfactant benchmark. PL was chosen since it has a relatively simple structure, and interference due its structure was likely to be negligible. PL is an anionic surfactant that has a relatively low lathering rate. In contrast, a surfactant such as sodium lauryl ether sulfate, commonly used in cleaning products, lathers easily and could confound the effects of adding an adjuvant surfactant.
The evaluation samples were prepared in a weight ratio of 85% primary surfactant 15% for each adjuvant surfactant to reflect levels similar to other foam-increasing agents used in commercial products. The pH of each sample was not adjusted so that the material adsorbed to the interface would be limited to surfactants. In fact, the pH of each sample stayed between 9 and 10, and no significant difference in pH between samples was observed.
DST, as measured in the time range of 50–5,000 msec by the maximum foam pressure method, showed a time-course decrease in the surface tension of the foams.3 However, to correlate DST results with the sensory evaluations, the researchers needed to establish changes in the DST during the first 100 to 1,000 msec, as established for the sensory evaluation. As measured by DST, the lower the surface tension value, the faster the adsorption of the sample to the air-water interface—i.e., the higher the lathering rate. The concentration of the sample in the aqueous solution provided a curve gradient, which is a critical factor to determining lathering rate when it is measured within this short period of time. The lower the concentration, as indicated by a large gradient, the longer the duration for saturation adsorption. If the concentration is too high, it becomes difficult to obtain significant information for an accurate comparison with the previous data due to the sample being in a near-saturation adsorption state.
The researchers experimentally optimized the concentration of the sample as 1% w/w. This type of measurement is generally conducted at a lower concentration than the critical micelle concentration (CMC) because the dispersion of monomer in the bulk solution is initially the dominant factor in the adsorption of the surfactant to the air-liquid interface. Nevertheless, this measurement intentionally was conducted above the CMC because the pre-experiment indicated that real use would not occur below the CMC, and the results would cover the observed effects on lathering rate.
Static surface tension: Static surface tension (SST) was measured by the Wilhelmy Plate Method, known as the CMC-derivation method. The same sample system as that used in the DST measurement was used; i.e., a combination of PL and an adjuvant surfactant, and the adjuvant surfactant alone.
Ross-Miles method (RMM): Increases in foam stability due to the addition of adjuvant surfactants were further evaluated by RMM. The concentration of the surfactants was set as 25% w/w at 40°C. Many commercially available surfactants having different carbon chain lengths were tested but only the adjuvant surfactants listed showed meaningful results. A comparative study of P2C8 and two other foam-increasing agents, cocamide MEAd and cocoyl methylethanol amide (cocamidemethyl MEA, or CMEA)e, was conducted and evaluated at 1% w/w by adding, to the cleanser base composed of potassium cocoate (PC)f, cocamidopropyl betaine (CAPB) and cocamide MEA in the ratio of 14:3:2, respectively.
Results and Discussion
The data from the sensory evaluations of the lathering rate and the foam volume of each sample is shown in Table 1. P2C8, capryloyldiethanolamide, capryloyl amidopropylcarbobetaine, capryloyl amidopropylsulfobetaine and capryloyl amidopropylamineoxide showed better results than LDA for foam volume, but P2C8 and caprylyl trimethylammonium chloride were better than LDA for lathering rate. Accordingly, only P2C8 scored positive in both categories. On the other hand, PEG-2 laurylamine (P2C12), a compound similar to P2C8, did not show the same performance as P2C8 in both categories. The decrease in carbon number of the alkyl chain from 12 to 8 apparently increases the lathering rate and the foam volume.
DST and Structure Relationship
Since P2C8 showed better results than other surfactant types, and P2C12 was similar to P2C8 in both lathering rate and foam volume, the researchers were interested in effects likely due to a relatively weak affinity between the hydrophilic group of the nonionic adjuvant surfactant and the anion group of PL; assumably, the low hydrophobicity of P2C8 would help to quickly respond to the newly formed air-water interface. Further, P2C8 has the same alkyl chain length as PL; in fact, seven derivatives of PEG-2-alkylamine, each having a different alkyl chain length—from C6 to C12, shown in Table 1—were synthesized using a known standard process4, 5 in order to determine optimum hydrophobicity.
Since DST was considered to provide the best understanding of the effect, the arithmetic averages of DSTs measured between 100 and 1,000 msec for each screened sample are shown in Figure 3. As described above, P2C8 is shown to have the largest lathering rate, indicating a proportional relationship with the DST. The surface tensions decreased from C6 to C8 and increased from C8 to C12. Also, the C8 derivative P2C8 had the highest lathering rate among the derivatives. A different factor or mechanism between lower and higher carbon numbers than C8 may affect the surface tension, as further described below.
The C6 and C7 derivatives, having lower interface activity, may suppress the adsorption to the air-water interface and thus lower the response despite dispersion in aqueous solution. On the other hand, C9–C12 derivatives are more repulsive against potassium laurate (PL), i.e., they have increased hydrophobic interaction due to their hydrocarbon length. The samples are listed in order of lathering rate; the smaller number reflects a greater rate. The differences in adsorption rates measured by DST were well-correlated to the in-house sensory evaluations. In addition, researchers found that if the difference was more than 3 mN/m in the dynamic region of DST, the difference in the sensory evaluation of lathering rate was unequivocal.
In general, the higher the hydrophobicity of a surfactant dispersing in water as a single component, the easier it is for adsorption to the air-water interface to take place.6 This is caused by the phenomenon whereby a hydrophobic alkyl chain sheds the contact area to water, and thus the hydrophobic chain is excluded from the water system. However, the relationship between the results shown in Figure 3 and the above mechanism is not straightforward. Although other factors may be involved, the following could serve as an explanation. If the adsorption of the adjuvant, e.g., P2C8, is faster than PL in the initial adsorption stage, the adsorption behavior could be monitored or measured; but if slower, it could not be observed. If the test was conducted on the adjuvant surfactant alone, the relationship between the length of the alkyl chain and the lathering rate was clearer. However, the adjuvant surfactant would not be used alone in commercial products, and the experimental observations of the adjuvant surfactant alone would not be reflected by the binary system.
In addition, not only the monomer but also the condition of the micelle could be involved since the concentration of surfactants in the binary solution was higher than CMC (see Table 2). As noted before, in accordance with elongation of the length of the alkyl chain, the hydrophobic interaction between the adjuvant surfactant and PL could become a significant factor. Specifically, the surfactants are aligned in the micelles as a mixed micelle so that the alkyl chain would hardly participate. From this evidence, it is considered that P2C8 might have the suitable proportion between hydrophobicity and hydrophilicity to provide the best lathering rate.
SST Measurement
SST was conducted to understand the action mechanism of P2C8 during lathering. The time-course foam volume of common surfactants and P2C8 are shown in Figure 4. The CMC value of P2C8 and related surfactants are shown in Table 2. P2C8 has a much higher CMC than P2C12, LDA and lauroyl propylene glycol. This high CMC value implies that the concentration of monomer in water is high even in a rather high concentration of the surfactant. In other words, the high concentration of monomer relative to adsorption to the air-water interface might contribute to increase the lathering rate as a critical factor.
The diffusion-controlled model (DCM), modeling an initial lathering stage, would be the most suitable among the few physical models proposed to explain the adsorption phenomena of surfactants to the air-water interface.7–10 Given there is no transition energy barrier on the monomer from the bulk layer to the surface layer, the concentration diffusion of the surfactant molecule is the driving force in DCM. This adsorption phenomenon complies well with DCM, and monomers of the adjuvant surfactant diffuse and easily adsorb to the air-water interface when the adjuvant surfactant is added. This has a significant impact on the lathering rate.
Stability of Foams
In addition to the ability to increase lathering rate, P2C8 showed significantly different properties of DST and SST than other surfactants. The stability of foams is important for the acceptance of a variety of consumer cleaning products not only for sensation, but also for cleaning purposes. The stability of foams produced by the formulations was tested by RMM. As shown in Figure 5, the test sample with P2C8 and the comparative samples with cocamide MEA (MEA) and cocamidemethyl MEA (CMEA) were prepared in the common formula including PC/CAPB/MEA. MEA and CMEA are generally known to increase foam volume, however P2C8 showed a significantly larger volume of foam at any point between 0 (immediately after application) and 30 min. These results obviously indicated that P2C8 provided larger foam volume than MEA and CMEA, and the volume decreased at a rate similar to those of MEA and CMEA.
The researchers propose the following action mechanisms, as shown in Figure 6, for P2C8 to increase the lathering rate and foam volume, and to maintain the foam volume. The addition of P2C8 increases the concentration of the monomer, and thus more molecules can adsorb to the air-water interface. As a result, adsorption saturation is quickly obtained, in which stable foams are quickly formed with less break down of foam—in other words, larger foam volume. In contrast, if the concentration of monomers were lower, the micelle must supply molecules and monomers and they would be irregularly distributed, resulting in corruption of the micelle. Accordingly, the lathering process would be inconsistent, the lathering rate would be lower, and the resulting foam volume, smaller.
Conclusion
P2C8 was found to increase the lathering rate as an adjuvant surfactant, and agents have been reported to increase lathering rate. Accordingly, the author proposes to call the new functional agent a “lather booster” or “foam booster.” P2C8 showed higher profiles in DST and SST as well as a foam volume, supporting the lather boosting effect. Among PEG-2-alkylamides, the hydrophobicity and hydrophilicity of P2C8 as an adjuvant surfactant are ideally balanced to serve as a lather booster. P2C8 also showed the same results even when formulated with other anionic surfactants and in compositions with silicones and higher alcohols, which generally suppress lathering. These details will be published in the future.
References
Send e-mail to [email protected].
1. T Sugiura, Concepts in basic bubble and foam engineering, Techno Systems Co, Ltd (2005) p 487
2. JIS K 3362, Test method of household synthetic detergent, Japanese Industrial Standards Committee, (Mar 20, 2008)
3. VB Fainerman, R Miller and P Joos, The measurement of dynamic surface tension by the maximum bubble pressure method, Colloid Polymer Sci 272, 731–739 (1994)
4. F Kanetani, K Negoro and H Takaishi, Synthesis and physicochemical and antimicrobial properties of N, N-Bis(2-hydroxyethyl)alkylamine oxides and some related compounds, The Chemical Society of Japan 9 1538–1545 (1982)
5. Y Ishii, The addition of ethylene oxide to the aliphatic amine, in Nonionic Surfactant (1965) p 33
6. MJ Rosen, Surfactants and Interfacial Phenomena, 3rd edn (2004) pp 34-104
7. AFH Ward and L Tordai, Time-dependence of boundary tensions. The role of diffusion in time-effects, J Chemical Physics 14 7 (Jul 1946)
8. J Eastoe and JS Dalton, Dynamic surface tension and adsorption mechanisms of surfactants at the air–water interface, Advances in Colloid and Interface Science 85(2–3) 103–144 (Mar 31, 2000)
9. VB Fainerman, AV Makievski and R Miller, The analysis of dynamic surface-tension of sodium alkyl sulfate-solutions, based on asymptotic equations of adsorption kinetic-theory, Colloids Surf A 87 61 (1994)
10. T Tamura and Y Kaneko, in S Hartland, ed, Surface and Interfacial Tension, Surfactant Science Series vol 119 (2004) pp 96–105