Editor’s note: Part I of this review appeared in October 2010. Part II appeared in January 2012, Part III is presented here, and the final Part IV appeared in May 2012.
Part I of this review on Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs) discussed the differences of these two delivery systems for cosmetic actives, as well as their production methods and selection criteria for constituents. In Part II, the characterization of these nano-sized particles was considered. In Part III, presented here, their stability and efficacy are considered; Part IV will address their application in cosmetics.
Active and Lipid Chemical Stability
The reason for including an active in a nanoparticle is not only to enhance skin delivery or controlled-release rate, but also, like liposomes and other deformable vesicles, to create a barrier to chemicals that may negatively influence the stability of the active. The following section deals with a couple of relevant cosmetic examples, illustrating how this can be investigated. The chemical stability of both the active and the lipid excipient can be at stake and are discussed separately here.
Active ingredient: Lipid nanoparticles have been shown to enhance the chemical stability of cosmetic actives such as vitamins1 and other compounds that are sensitive to light, oxidation and hydrolysis. However, this effect is not always straightforward. When Jenning and Gohla compared the chemical stability of all-trans retinol incorporated in SLNs with an emulsion, enhanced stability was expected from the SLNs but the retinol was stabler in the emulsion.2 Only after the researchers reduced the retinol loading and increased its solubility in the SLN by adding extra oil to the lipid—in fact by creating an NLC, a term that had not yet been coined—were they able to create a stabler form of retinol than the microemulsion.2 This is shown in Figure 1.
Another series of experiments investigating the effect of formulation parameters on the chemical stability of ascorbyl palmitate was conducted by Teeranachaideekul et al.3 The researchers explored different types of lipids, surfactants, antioxidants, storage conditions—i.e., temperature and nitrogen gas flushing, as well as the effects of drug loading. Just like Jee et al. had shown for retinol,4 the addition of antioxidants improved the stability of ascorbyl palmitate. After storage for 90 days in NLCs containing a high loading of ascorbyl palmitate, more than 90% active remained after flushing with nitrogen gas, adding a combination of antioxidants—butylate hydroxyanisol (BHA), butylate hydroxytoluene (BHT) and DL-α-tocopherol (vitamin E)—and storage at 4°C.3 But the fact remains: conditions differ from active to active.
Lipid excipients: There are few reports in the literature that describe the chemical stability of lipids used in lipid nanoparticles, and the only paper dealing specifically with this subject is quite extensive. Radomska-Soukharev investigated various lipids and surfactants.5 SLNs of varying compositions were made, differing mainly in their relative percentages of mono-, di- and triglycerides, and stored for 24 months. The lipid content dropped from 100% immediately after SLN production via hot homogenization and, after two years, reached around 90–95%. The SLNs obtained with triglycerides were more stable than those composed of mono- and diglycerides. In the case of SLNs with a triglyceride content of 97%, the chemical stability was the highest (> 96%) after 2 years, whereas the stability of SLNs with a mono- and diglyceride content of 95% was the lowest (~ 89–95%) after 2 years.5 Relative to the chemical stability of the active, the chemical stability of the lipid is only of minor concern.
Efficacy of SLNs and NLCs
One of the main differences between a nanoparticle containing an active and an emulsion containing the same is the rate at which the active is released to the skin and becomes available for absorption. Therefore, the release rate and the skin penetration of actives from SLNs and NLCs will be considered next, in comparison with emulsions. In addition, two benefits from nanoparticles based on their occlusivity will be discussed; i.e., skin moisturization and sun protection.
Release rate of actives: As discussed in Part 1 of this article series, the active/ lipid ratio determines the location of the active within the nanoparticle. If the ratio is optimal, the lipid and active precipitate at the same time during the cooling phase of the production process, resulting in a uniform distribution of the active. If the amount of lipid is too high, the lipid precipitates first, resulting in an active-enriched outer shell. On the other hand, if the amount of active is too high, the active precipitates first and the core is active-enriched. These three different forms result in different release profiles, respectively: a constant release, a burst release and a retarded release. Relative to an emulsion, however, the release of actives from nanoparticles is significantly retarded, as illustrated in Figure 2.
While the active and lipid and their relative and absolute concentrations determine the release rate of the active, large variations may still occur. In fact, zur Mühlen et al.8 found a 100% release of tetracaine and etomidate within 1 min from SLNs, whereas prednisoloneloaded SLNs showed a controlled release over a period of five weeks. In this study, the lipid and surfactants were the same; only the level of surfactant was halved and a different active was used. On top of these variables, the release rate also depends on the production method, as smaller particles demonstrate higher release rates than larger particles.8 This is easily understood from the surface area/gram lipid ratio.
Skin penetration of actives: The release of an active from an SLN only indicates the amount of active that reaches the skin surface as a function of time; it does not guarantee efficacy. Penetration of active-loaded nanoparticles into the skin must also be considered. However, a recent publication examining cosmetic nanoparticles revealed that inorganic sunscreen particles do not penetrate the skin. Inorganic engineered nanoparticles like titanium dioxice and zinc oxide were occasionally found within the stratum corneum, but did not penetrate into the living layers of the skin.9 Similarly, polymeric nanoparticles were shown to remain on the skin surface or enter the infundibulum, but only if massaged into the skin.10
The SLNs and NLCs discussed here are different from the “hard” and “inert” inorganic materials that make up sunscreens and polymeric nanoparticles. This raises the question of whether they penetrate skin. Baroli gave three reasons why lipid nanoparticles may enhance skin delivery. 1) Ultra-deformable particles like ethosomes and transfersomes may be able to enter into the intercorneocyte space; however, SLNs and NLCs are not ultra-deformable, so this reason will not be discussed further. 2) Lipid nanoparticles may enhance skin delivery because they sit or melt on the skin surface, thus increasing skin hydration via occlusion (see Occlusivity of SLNs and skin hydration). Finally, 3) constituents of the nanoparticles may enter the stratum corneum and disorganize the lipid matrix that makes the skin barrier.11
Alvarez-Román et al. give another logical reason, namely an altered thermodynamic activity and therefore an increase in the partition coefficient, which was demonstrated for the dye Nile Red.14 Considering the plethora of scientific articles describing SLNs and NLCs, it is remarkable that only a few actually measure the skin penetration of their payload. Table 1 (downloadable in two parts, PDF 1 and PDF 2) lists all publications that describe the enhanced skin delivery achieved via SLNs and NLCs. Molecules (i.e., drugs or cosmetically active ingredients) are listed alphabetically and if more than one publication exists, listed chronologically.
In all these studies, the delivery obtained from SLNs or NLCs was compared to the delivery of the same active on skin of the same animal or person from a conventional emulsionbased vehicle. Very often, but not always, a nano-emulsion was used in an attempt to mimic the particle size of the nanoparticles. The delivery of the active or drug from the emulsion-based vehicle was set as unity so that the enhancement ratios could be calculated as shown in Eq. 1.

Eq. 1
Where possible, the enhancement ratio has been calculated from the data provided in the articles but occasionally it could only be estimated from figures in these articles. Some conclusions can be drawn.
1) On average, the enhancement ratios of SLNs are higher than NLCs; most likely due to the higher thermodynamic activity of the incorporated drug or active at the same concentration, as was shown above in studies comparing SLNs and NLCs.
2) The enhancement ratio depends on the depth within skin where the amount of drug or cosmetic active ingredient is measured. Toward the top of the skin, the enhancement is approximately 4, which is higher than in the deeper layers of the skin where the enhancement ratio decreases to approximately 2. In general, the deeper layers contain even less material than is delivered from an emulsion.
3) The enhancement ratio also depends upon the time at which the measurement is taken. Most measurements of drugs or actives in the skin were taken at 6 hr and 8 hr, resulting in reasonable enhancement ratios. As time goes by, the enhancement ratio drops and likewise, the error in the ratio increases as the absolute numbers decrease.
4) In the majority of studies, glyceryl behenate and glyceryl palmitostearate were used to create the SLNs with either oleic acid or caprylic/capric triglycerides added in case NLCs are made. And the variation in surfactants is minimal, which could be an explanation for the limited range in enhancement factors; it may be possible that larger or smaller enhancement ratios could be obtained with diff erent SLN and NLC constituents.
In all, two types of analysis were used to evaluate potentially enhanced delivery—i.e., microscopic observation using model dyes, and actual measurements of penetrated drug amounts, which account for incorporated as well as free drug or active amounts. Examples of these two types of analysis are shown in Figure 3 and Figure 4. Figure 3 beautifully illustrates the enhanced delivery of Nile Red from SLNs and NLCs following 8 hr of passive penetration.15 It can be clearly seen that the delivery of the dye is greater from the SLN (Figure 3b), followed by the two types of NLCs (Figure 3c-d) relative to the same amount of Red Nile in a conventional cream (Figure 3a). Finally, Figure 4 shows the distribution of the drug cyproterone acetate, separated out in layers of 100 μm, and confi rms that the delivery is increased in the top layers of the skin.
Occlusivity of SLNs and skin hydration: While many review papers on the topic of SLNs note their occlusive properties, original research on this subject is quite limited. Th is line of research started during the doctoral theses of Jenning and Dingler, and was the main subject of the doctoral thesis of Wissing, which are described here.
It is assumed that SLNs and NLCs form fi lms of densely packed spheres on the surface of the skin that exert an occlusive eff ect.18 Figure 5 illustrates an electron micrograph of an air-dried SLN dispersion and shows that the lipid nanoparticles do indeed merge.23 This leads to increased hydration and subsequently, to the smoothing of wrinkles—an eff ect utilized in many cosmetic products. Jenning originally used three different techniques to demonstrate the occlusive properties of SLNs: transepidermal water loss, microscopy and increased skin penetration of retinyl palmitate—an “occlusion-sensitive” molecule.18 However, around the same time, this approach was replaced by measurement of the occlusion factor.24 In this assessment, 100-mL beakers are filled with 50 mL water and sealed with a cellulose acetate filter. The samples of which the occlusivity is to be measured are spread on the filter (13.3 mg/cm2) and stored at 32°C and 50–55% relative humidity for 48 hr. The occlusion factor F is calculated using Eq. 2 after 6 hr, 24 hr and 48 hr, where A is water loss without the sample (reference) and B is the water loss with the sample.25
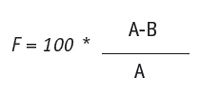
Eq. 2
The most extensive work on the occlusion aspect of solid lipid nanoparticles was conducted by Wissing et al., who investigated the effects of particle size, applied sample volume, lipid concentration and the crystallinity of the lipid matrix.26 It was found that up until a particle size of 400 nm, the occlusion factor F was constant but with larger particles, it dropped. Occlusivity also increased with increasing sample volume up until 200 mg, where it started to plateau. Lipid concentrations were increased from 5% to 40% and rose in line with concentration until a plateau was reached at 35% lipid. Finally, when going from a supercooled melt to a highly crystalline lipid matrix, the occlusivity increased accordingly and the occlusivity of an SLN, when compared with an emulsion, was somewhat but statistically significantly higher. The researchers concluded that the conditions for optimized occlusivity are a particle size of about 200 nm, a minimum lipid mass of 3.7 mg/cm2, and a high degree of crystallinity of the lipid matrix.26
Figure 6 shows why nanoparticles do indeed cause occlusion while microspheres do not. While the scientific literature claims this to be due to a denser packing of smaller particles, another theory could explain the occlusivity. The “free” space, i.e., the total surface area of a square minus that of the largest circle fitting into this square, remains constant independent of the radius, thus the total surface area available from which water can escape from the skin surface is constant. However, if the volume of free space also is calculated, an eightfold reduction is obtained when the radius is halved. Since the experiments to investigate the influence of particle size were conducted at a constant lipid content, this implies that in contrast to what is shown in schematic explanations of this occlusivity phenomenon (see Figure 6a-b), multiple layers of particles exist. As a consequence, the efficiency of packing these layers—the the so-called “close packing” of spheres—is also playing a role. This could be shown by demonstrating that nanoparticles with a high PI have a lower occlusivity factor than nanoparticles with a small PI.
The consequence of nanoparticles occluding human skin has been convincingly shown in in vivo experiments.27–29 The effect of SLNs on skin hydration was demonstrated in a blind, placebo-controlled study involving 25 volunteers. Formulation A was an o/w cream and formulation B was an o/w cream enriched with 4% SLN. Subjects applied the creams twice daily for four weeks on their volar forearms and skin hydration was measured with a corneometer after two and four weeks. Both creams significantly increased skin hydration, in comparison with untreated control sites. Skin hydration using formulation A was 21% and 24% at week two and four, respectively; for formulation B, the increases were 29% and 32%. From this, it can be concluded that the addition of SLNs to formulation A would result in an additional increase of 38% and 33% at week two and four, respectively.29
Sun protection: A last benefit of SLNs to discuss is their action as a physical sunscreen. Since the particulate matter in SLNs is a solid material, they could, in principle, act in this way. Wissing and Müller were the first to investigate this and concluded that the concentration of benzophenone-3 could be halved without the loss of protection efficacy.12, 30, 31 This conclusion was later extended to other sunscreens such as ethylhexyl methoxycinnamate and butyl methoxydibenzoylmethane.32 The researchers concluded that this is due to the sustained release of the sun filter itself, keeping the molecular sunscreen on the skin surface for longer, as well as the SLN acting as a physical sunscreen itself.
Concluding Remarks, Part III
The characterization of SLNs and NLCs offers many advantages. Due to the establishment of the relationship between particle properties and their performance, preferred ranges for these parameters can be clearly indicated, making faster product development possible. Issues such as particle size, particle size distribution and crystallinity must be determined, and they predict the performance of these nanoparticles. The main skin penetration enhancement ratio is between 0.7 (a small retardation) and 4 (a mild enhancement). Remarkably, the fact that this happens without any signs of skin irritation is something that is not stressed in the scientific literature but very important for developers of cosmetic products.
The next and last part of this four-part review of SLNs and NLCs in cosmetics will deal with marketed products containing these lipid-based nanoparticles.
References
- J Pardeike, A Hommoss and RH Müller, Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products, Int J Pharm 366 170–184 (2009)
- V Jenning and SH Gohla, Encapsulation of retinoids in solid lipid nanoparticles (SLN), J Microencaps 18 149–158 (2001)
- V Teeranachaideekul, RH Müller and VB Junyaprasert, Encapsulation of ascorbyl palmitate in nanostructured carriers (NLC)– Effects of formulation parameters on physical stability, Int J Pharm 340 198–206 (2007)
- J-P Jee, S-J Lim, J-S Park and C-K Kim, Stabilization of all-trans retinol by loading lipophilic antioxidants in solid lipid nanoparticles, Eur J Pharm Biopharm 63 134–139 (2006)
- A Radomska-Soukharev, Stability of lipid excipients in solid lipid nanoparticles, Adv Drug Del Rev 59 411–418 (2007)
- EB Souto, SA Wissing, CM Barbosa and RH Müller, Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery, Int J Pharm 278 71–77 (2004)
- M Joshi and V Patravale, Formulation and evaluation of nanostructured lipid carrier (NLC)-based gel of valdecoxib, Drug Devel Ind Pharm 32 (2006) 918–918.
- A zur Mühlen, C Schwarz and W Mehnert, Solid lipid nanoparticles (SLN) for controlled drug delivery–Drug release and release mechanism, Eur J Pharm Biopharm 45 149–155 (1998)
- JW Wiechers and N Musee, Engineered inorganic nanoparticles and cosmetics: Facts, issues, knowledge gaps and challenges, J Biomed Nanotechnol 6 408–431 (2010)
- J Lademann et al, Nanoparticles–An efficient carrier for drug delivery into the hair follicles, Eur J Pharm Biopharm 66 159–164 (2007)
- B Baroli, Skin absorption and potential toxicity of nanoparticulate nanomaterials, J Biomed Nanotechnol 6 485–496 (2010)
- SA Wissing and RH Müller, Solid lipid nanoparticles as carrier for sunscreens: In vitro release and in vivo skin penetration, J Contr Rel 81 225–233 (2002)
- J Štecová et al, Cyproterone acetate loading to lipid nanoparticles for topical acne treatment: Particle characterization and skin uptake, Pharm Res 24 991–1000 (2007)
- R Alvarez-Román, A Naik, YN Kalia, RH Guy and H Fessi, Enhancement of topical delivery from biodegradable nanoparticles, Pharm Res 21 1818–1825 (2004)
- S Lombardi Borgia et al, Lipid nanoparticles for skin penetration enhancement–Correlation to drug localization within the particle matrix as determined by fluorescence and parelectric spectroscopy, J Contr Rel 110 151–163 (2005)
- S Küchler et al, Nanoparticles for skin penetration enhancement–A comparison of a dendritic core-multishell-nanotransporter and solid lipid nanoparticles, Eur J Pharm Biopharm 71 243–250 (2009)
- C Santos-Maia, W Mehnert and M Schäfer- Korting, Solid lipid nanoparticles as drug carriers for topical glucocorticoids, Int J Pharm 196 165–167 (2000)
- J-Y Fang, C-L Fang, C-H Liu and Y-H Su, Lipid nanoparticles as vehicles for psoralen delivery: Solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC), Eur J Pharm Biopharm 70 633–640 (2008)
- V Jenning, A Gysler, M Schäfer-Korting and SH Gohla, Vitamin A loaded solid lipid nanoparticles for topical use: Occlusive properties and drug targeting to the upper skin, Eur J Pharm Biopharm 49 211–218 (2000)
- PV Pople and KK Singh, Development and evaluation of topical formulation containing solid lipid nanoparticles of vitamin A, AAPS PharmSciTech 7(4) article 91, E1–7 (2006)
- EG de Jalón, MJ Blanco-Príeto, P Ygartua and S Santoyo, PLGA microparticles: Possible vehicles for topical drug delivery, Int J Pharm 226 181–184 (2001)
- VB Junyaprasert, V Teeranachaideekul, EB Souto, P Boonme and RH Müller, Q10- loaded NLC versus nanoemulsions: Stability, rheology and in vitro skin permeation, Int J Pharm 377 209–214 (2009)
- RH Müller, M Radtke and SA Wissing, Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations, Adv Drug Del Rev 54 suppl. 1 S131-S155 (2002)
- A Dingler, RP Blum, H Niehus, RH Müller and S Gohla, Solid lipid nanoparticles–A pharmaceutical and cosmetic carrier for the application of vitamin E in dermal products, J Microencaps 16 751–767 (1999)
- SA Wissing and RH Müller, The influence of the crystallinity of lipid nanoparticles on their occlusive properties, Int J Pharm 242 377–379 (2002)
- SA Wissing, A Lippacher and RH Müller, Investigations on the occlusive properties of solid lipid nanoparticles (SLN), J Cosmet Sci 52 313–324 (2001)
- SA Wissing and RH Müller, The influence of solid lipid nanoparticles on skin hydration and viscoelasticity–in vivo study, Eur J Pharm Biopharm 56 67–72 (2003)
- RH Müller, RD Petersen, A Hommoss and J Pardeike, Nanostructured lipid carriers (NLC) in cosmetic dermal products, Adv Drug Del Rev 59 522–530 (2007)
- SA Wissing and RH Müller, Cosmetic applications for solid lipid nanoparticles (SLN), Int J Pharm 254 65–68 (2003)
- SA Wissing and RH Müller, The development of an improved carrier system for sunscreen formulations based on crystalline nanoparticles, Int J Pharm 242 373–375 (2002)
- SA Wissing and RH Müller, Cosmetic applications for solid lipid nanoparticles (SLN), Int J Pharm 254 65–68 (2003)
- Q Xia, A Saupe, RH Müller and EB Souto, Nanostructured lipid carriers as novel carrier for sunscreen formulations, Int J Cosmet Sci 29 473–482 (2007)












