Prolonging the degradation and volatilization of fragrances is key to creating personal care products with extended and balanced sensory stimuli. Encapsulation is a widely used approach for controlling the delivery of sensory compounds and other active ingredients. In addition to altering the rate of fragrance vaporization, encapsulation can be beneficial for formulations where the volatile compound(s) are insoluble in a suitable solvent system.
Various encapsulation technologies have been developed, including entrapment within polymer systems,1-3 molecular inclusion in a host such as cyclodextrin,4 absorption into silica microspheres5 and co-addition to various emulsified fluids prepared via coacervation.6, 7 Another is the loading of active ingredients into polymers via interfacial polymerization or in situ polymerization.8 The fundamental principle of delivery is to limit the rate of fragrance diffusion through hindered mass transport. Binding the fragrance to a surface or host may also play an important role in reducing volatility and extending the sensory characteristics of the product; this has been explored by some.9 Regardless of the approach, fragrances must be chemically inert to be encapsulated by most mechanisms, especially those that use active polymerization routes to entrap solutes.
The stimulated release and increase in delivery of fragrances during physiological or environmental changes is also a mechanism of interest. In relation, chemistries have been developed that link pro-fragrance molecules to a solid support through a liable covalent bond.10 Without a stimulus, the pro-fragrance is incapable of being volatized by the covalent bond linkage; however, bond breakage and volatilization of the fragment can be induced by light,11 heat,12 hydrolysis,13 changes in pH14 or the activity of enzymes.15 A drawback to chemical bond cleavage and delivery is the limited number of mild reactions and pro-fragrances that are available. Also, careful packaging is necessary to extend shelf-life of the inherent reactive pro-fragrance systems. Further research is needed to optimize delivery systems, increase the number of simultaneously released fragrances, and reduce the need for expensive raw material stocks.
Other options for potential fragrance delivery are animated materials that can change their physical properties as a function of a stimulus. Hydrogels, which can swell with water, are the most common example of such materials, changing their porosity to deliver an active ingredient.16, 17 Through careful control of their chemistry, hydrogels can be made to be thermally reversible18, 19 or pH-responsive.20, 21 Silica-based solids typically are characterized as inelastic and not swellable, with only rare exceptions.22, 23 For other materials, a modest degree of swelling has been reported, typically < 15% of their initial volume, based on some type of physical or chemical change; for example, a change in temperature or pH. Described here is a chemically inert nanoporous organosilicaa that is designed to rapidly (< 1 sec) swell up to four times its volume and eight times it mass with organic solvents. Here, the author evaluates its capabilities for the extended release of volatile fragrances and the stimulated release of active ingredients.
Nanoporous Organosilica Mechanism
The functionality of the described nanoporous organosilica arises from a matrix consisting of flexibly tethered organosilica particles with diameters of ~20 nm arranged in a disorganized network. The expanded state, under solvent-saturated conditions, is the low energy or relaxed matrix arrangement. The dry or collapsed matrix state, induced by capillary collapse during solvent evaporation, leads to bond deformations and limited degrees of freedom resulting from the tensioning of the matrix.
Figure 1 (top) shows the nanoporous organosilica swelling upon the drop-wise addition of acetone. Across the middle are SEM images of the nanoporous organosilica matrix in the dry collapsed form; in a partially swollen state, captured by swelling the matrix with a poly(2,2,3,3,4,4,4-heptafluorobutyl methacrylate) solution and allowing the solvent to dry, leaving the polymer entrapped; and (right) fully expanded as captured by swollen in ethanol, followed by critical point drying. The bottom image is a schematic representation of the arrangement of organosilica nanoparticles in the corresponding swollen states.
Intermolecular forces between the collapsed surfaces (500-900 m2/g) retain the system in a tensioned state in the absence of absorbates, including all small molecule non-polar and semi-polar organics. When inter-particle non-covalent interactions are disrupted by the absorption of organics, this potential energy stored within the matrix architecture is released. The high degree of interconnectivity within the pore structure allows the relaxation process to happen rapidly in the presence of organic liquids and vapors. The swelling process is slightly endothermic (5.2 ± 1.2) J/g, indicating that expansion is entropically favorable. As absorbates are bound and the material changes volume, the porosity and mass transfer rates increase, allowing for encapsulated molecules to enter or exit the material more readily.
The animated organosilica described consists of polycondensed alkoxysilane precursors that contain a bridging organic group possessing an aryl ring. This aromatic aryl group allows for π-π stacking, enabling the molecular self-assembly of the nanoparticles that cross-link and thus comprise the matrix. Careful processing steps are required to create a system that is flexible yet macroscopically resembles a transparent solid. For personal care applications, monolithic material is ground to a powder, which does not affect the functional characteristics. Following polycondensation and post-processing, the final material is chemically inert and has textural and chemical properties similar to polydimethylsiloxane powder. To test the effectiveness of the described structure and mechanisms, the nanoporous organosilica was evaluated for extended and stimulated release.
Materials and Methods
Materials: The nanoporous organosilica was prepared as described previously24 using hexamethyldisilazane as a post-polymerization derivatization agent. Bulgarian rose extractb was chosen as a model fragrance concentrate with volatile components that rapidly evaporate. Other chemical reagents (menthol, ethanol and lidocaine)c also were obtained.
Encapsulation: One advantage of the nanoporous organosilica is its ease of loading; fragrances do not require co-polymerization during material synthesis. Instead, they can be added by dissolving the compounds in any suitable low boiling-point organic solvent and then adding the solution to the preformed solid. Swelling will allow the liquid, including odorous solutes, to be entrained within the nanoporous network. The solvent can then be removed by evaporation at room temperature, leaving solutes entrapped in the matrix.
Rose extract was diluted 1:20 in dichloromethane. The solution was added to the nanoporous organosilica until the material was fully swollen (~ 5.5 mL/g), as determined visually. The dichloromethane was allowed to evaporate at room temperature under a stream of nitrogen, which required 15-20 min.
Controls were prepared by depositing and subsequently evaporating the same amount of solvent onto silanized glass beads and natural sponge. A solution of 5% w/v hexanol, dodecane and menthol was also prepared in dichloromethane and loaded by swelling into the nanoporous organosilica, then deposited onto glass beads and natural sponge. Loading three compounds allowed for the study of release rate vs. chemical-physical properties. Finally, a 0.1% w/v solution of lidocaine in ethanol was loaded into the nanoporous organosilica using the swell and solvent evaporation method.
Fragrance delivery measurements: The concentrations of volatile odorants over time were measured via headspace gas chromatography/mass spectrometry (GC/MS). Between measurements, 100-mg samples were stored in open vials in a fume hood with an air velocity of 100 ft3/min. After a set desorption time, the vials were sealed and equilibrated, following which 500 μL of headspace was injected onto a mass selector detectord. Analytes were detected using selected ion monitoring.
Lidocaine delivery: A 100-mg sample of the nanoporous organosilica with encapsulated lidocaine was placed in a column and connected to an HPLCd system. The mobile phase consisting of water or water with ethanol was varied, and the desorption of lidocaine was measured using a UV detector at 220 nm.
Results: Extended Delivery of Rose Extract
The delivery rate of encapsulated liquid odorants was evaluated using rose extract. The chemical composition of the extract was first analyzed by GC/MS. The major odorous compound was determined to be a-pinene (bp = 155°C), with the minor components camphene, b-pinene, limonene, octadiene, octatriene and 3,7-dimethyl-octen-1-ol. Desorption kinetics were measured using a-pinene, chosen for quantitation due to its much higher abundance. The amount of a-pinene was measured in the headspace over time for rose extracted loaded into the nanoporous organosilica, as compared to glass beads and natural sponge (see Figure 2).
Overall, the amount of a-pinene in the headspace above the glass beads and natural sponge was greater—seven times that of the nanoporous organosilica—at the first two-hour sampling point. This rapid release depleted the amount of odorant in the glass beads and natural sponge so that by 12 hr, no odorant was detectable above the glass beads or natural sponge.
In contrast, after four hours, a-pinene desorption from the nanoporous organosilica reached a near steady rate. Pinene was still detectable by GC/MS after 12 hr. Prior to the near steady-state desorption of a-pinene from the nanoporous organosilica, an approximate tenfold decrease in headspace concentration was observed, indicating a faster release at early times. This higher initial rate of volatilization is presumed to be due to the release of fragrance from the surface or near-surface region of the swellable material.
Upon loading, higher amounts of fragrances were likely concentrated near the surface of the absorbent particles via transport and deposition as the solvent evaporated from the surface pores. This heterogeneous distribution of loaded fragrances may be difficult to prevent unless there is a strong anchoring mechanism, such as adsorption to internal surfaces. However, the ease of loading is a substantial advantage despite the lack of full control over spatial distribution of the fragrance molecules in the final collapsed state of the material.
Results: Extended Delivery of Menthol
The delivery rate of encapsulated solid odorants was evaluated using menthol (mp = 37°C, bp = 212°C). Hexanol (bp = 156°C) and dodecane (bp = 215°C) were co-entrapped to compare the release rates of odorants that would be liquid phase in pure form at room temperature. The rate of release for all three compounds was measured at 25°C over a period of 24 days (see Figures 3, 4 and 5).
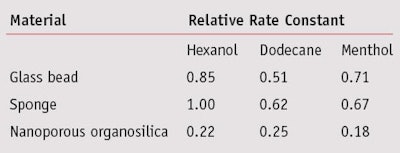
Menthol volatilization from nanoporous entrapment was found to be slower than from natural sponge or glass beads. Assuming volatilization followed the first order kinetics, the rate constant for menthol was 3.7–3.9 times less than for the glass beads and natural sponge (see Table 1). After 24 days, the relative amount of menthol released into the headspace was 47 times greater from the nanoporous organosilica than natural sponge. This indicates the possibility of a greater odorant reservoir being yet available in the organosilica after the elapsed period of time.
Comparing the release rates of the three co-entrapped, semi-volatile compounds, the rate constant for appearance in the gas phase was relatively constant across the set when encapsulated in the nanoporous organosilica. In contrast, the rate of evaporation for the compounds from the glass beads and sponge was closely related to their respective boiling points, e.g., equilibrium vapor pressure.
Results: Stimulated Release of Lidocaine
The release of encapsulated lidocaine (2-(diethylamino)-N-(2,6–dimethylphenyl)acetamide was measured in response to changes in aqueous phase ethanol concentration, which served as a stimulus to induce partial swelling. Lidocaine was chosen as it has low volatility and is relevant for personal care applications. A continuous flow system was used whereby an aqueous solution could be passed across lidocaine-loaded nanoporous organosilica. The composition of the aqueous phase could be varied and the concentration of lidocaine measured in real-time using a downstream UV detector (see Figure 6). Human skin emits a variety of volatile organic metabolites25 in addition to oil. Many of the volatile compounds are odorous, so absorption of such compounds with concomitant release of an active ingredient or fragrance would be desirable. Future work is yet required to examine delivery under in vivo conditions.
When a pH 7 buffer water was used, an initial release of ~ 4% of the loaded lidocaine was observed, presumably due to rinsing material localized to the surface of the particles. After the initial removal of these residues, only a small amount of lidocaine (< 0.01 ppm) continued to be released when the solution was buffered with water. The composition of the mobile phase was varied by creating three-minute durations of 5% v/v ethanol to act as a swelling stimulus.
Significant release of lidocaine was observed upon stimulus with dissolved ethanol, however, when the aqueous phase was returned to pure buffer, the release of lidocaine significantly diminished. The process was repeatable, although smaller amounts of lidocaine were released upon each stimulus, presumably due to depletion of the total reservoir of active ingredient. Continued stimulated released combined with calibration measurements indicated that > 90% of the encapsulated lidocaine could be delivered to the aqueous phase. As the concentration of the dissolved ethanol stimulus increased, a higher release of lidocaine was observed (see Figure 7).
The mechanism for the enhanced rate of encapsulated molecule release is hypothesized to be facilitated by two processes that increase mass transport efficiency. The first process is pore-opening, induced by the absorption of ethanol by the nanoporous organosilica matrix, which leads to a less constricted nanoporous architecture. The second process is speculated to be increased diffusion rates by absorbed ethanol dissolving encapsulated lidocaine, which otherwise exists as a solid at room temperature and inherently has limited or no diffusivity.
Absorption and swelling of the material are completely reversible, therefore when the ethanol concentration decreases in the aqueous phase, absorbed ethanol leaves the matrix as equilibrium is re-established, closing the pore structure and removing the absorbed solvent that facilitates transport out of the nanoporous particles. Although these experiments were conducted using lidocaine as the encapsulated molecule, additional experiments on a variety of other active ingredients show similar release dynamics (data not shown).
Ethanol in water was used as a stimulant to allow quantitative measurements of release using an analytical chromatography system under continuous flow to deduce the mechanism. Absorption by organosilica is semi-specific, and a similar effect is expected for any small non-polar or semi-polar organic compound, including oils and volatiles found in sweat. It is acknowledged that further work must be conducted to extend these results to in vivo conditions at relevant concentrations.
Formulating Considerations
Nanoporous organosilica is a fine powder that can be successfully incorporated with other dry ingredients such as iron oxide, clay, starches, silica, talc and magnesium stearate. The material cannot be used in conjunction with organic liquids or non-polar polymers as these will be absorbed, induce swelling and alter the characteristics of the formulation. It should be noted that swelling is reversible, meaning that when the organic liquid evaporates, the nanoporous silica will be left in the dry and collapsed state. Delivery by an alkane fluid spray is possible, leaving dry material.
Loaded nanoporous organosilica can also be dispersed in aqueous solutions. Emulsified systems could be impacted by addition of the material due to absorption of certain co-ingredients. Also, the organosilica is chemically inert, so the shelf-life of fragrances and actives should not be diminished, and may be improved by sequestration. Further, the material is not affected by freezing or exposure to elevated temperatures up to 250°C.
Discussion and Conclusions
The described nanoporous organosilica is of interest as a new type of encapsulant for fragrance and active ingredient delivery. One feature is the ease with which a small molecule can be loaded into the material by swelling, which enables the organosilica to absorb large volumes of solutes in organic solvents. Evaporation of the solvent then leaves semi-volatile components entrapped.
The reduction in release rates from the nanoporous organosilica appears to be primarily due to limiting mass transport by requiring molecules to diffuse through tortuous pathways in the nanoporous matrix. The surface chemistry is hydrophobic and generally inert, and does not seem to play a role in limiting release—although it is interesting to consider how decorating the pores with organic functional groups would lead to a specific and higher energy surface adsorption that could also alter release dynamics. Stimulation by absorption of endogenous organic molecules may be useful for products that are designed to respond under certain conditions.
While nanoporous organosilica is chemically inert, biocompatible and similar in chemical composition to polydimethysiloxane, which is a common ingredient in the personal care industry, the material is new to personal care so its regulatory status has yet to be determined. It is in the early stages of implementation for cosmetics and hair care applications, although the material has current applicability in the home care and water purification markets, meeting regulatory guidelines in these areas. Nanostructured organosilicas offer a new approach to controlled release in the cosmetics industry pending approval for market deployment.
Acknowledgements: The author wishes to thank Caitlin Scott for assistance with the described rose extract experiments and Emily Linville for assistance with the lidocaine release experiments.
References
1. JS Hwang, Preparation and characterization of melamine-formaldehyde resin microcapsules containing fragrant oil, Biotechnol Bioprocess Eng 11 332–336 (2006)
2. WO 98/13136 A1, Method for preparing microcapsules of active substances coated with a polymer and novel microcapsules in particular resulting from the method, B Jean-Pierre, R Joel and T Curtis, (1998)
3. A Sansukcharearnpon, S Wanichwecharungruang, N Leepipatpaiboon, T Kerdcharoen and S Arayachukeat, High loading fragrance encapsulation based on a polymer-blend: Preparation and release behavior, Intl J Pharmaceutics 391, 267–273 (2010)
4. CX Wang and SL Chen, Fragrance-release property of b-cyclodextrin inclusion compounds and their application in aromatherapy, J Ind Text 34, 157–166 (2005)
5. P Wang, Y Zhu, X Yang and A Chen, Prolonged-release performance of perfume encapsulated by tailoring mesoporous silica spheres, Flavour and Frag J 23, 29–34 (2008)
6. A Edris and B Bergnståhl, Encapsulation of orange oil in a spray dried double emulsion, Food / Nahrung 45 133–137 (2001)
7. AR Bachtsi and C Kiparissides, Synthesis and release studies of oil-containing poly(vinyl alcohol) microcapsules prepared by coacervation, J Controlled Release 38 49–58 (1996)
8. DL Berthier et al, Controlled release of volatile fragrance molecules from peo-b-ppo-b-peo block copolymer micelles in ethanol−water mixtures, Langmuir 26(11) 7953–7961 (2010)
9. CP Chang and T Dobashi, Preparation of alginate complex capsules containing eucalyptus essential oil and its controlled release, Colloids Surf B: Biointerfaces 32 257–262 (2003)
10. A Herrmann, Controlled release of volatiles under mild reaction conditions: From nature to everyday products, Angew Chem Int Ed 46 5836–5863 (2007)
11. A Herrmann, C Debonneville, V Laubscher and L Aymard, Dynamic headspace analysis of the light-induced controlled release of perfumery aldehydes and ketones from a-keto esters in body care and household applications, Flavour and Fragrance J 15, 415–420 (2000)
12. Y Houminer, Studies on the thermolysis of 2-(2-hydroxy-2-arylethyl)pyrazines. An example of a retro-ene-type reaction, J Org Chem 45(6) 999–1003 (1980)
13. B Levrand, W Fieber, JM Lehn and A Herrmann, Controlled release of volatile aldehydes and ketones from dynamic mixtures generated by reversible hydrazone formation, Helvetica Chimica Acta 90, 2281–2314 (2007)
14. JY de Saint Laumer, E Frérot and A Herrmann, Controlled release of perfumery alcohols by neighboring-group participation. Comparison of the rate constants for the alkaline hydrolysis of 2-acyl-, 2-(hydroxymethyl)-, and 2-carbamoylbenzoates, Helv Chim Acta 86(8) 2871–2899 (2003)
15. V Athawale, N Manjrekar and M Athawale, Lipase-catalyzed synthesis of geranyl methacrylate by transesterification: Study of reaction parameters, Tetrahedron Lett 43(27) 4797–4800 (2002)
16. Y Qiu and K Park, Environment-sensitive hydrogels for drug delivery, Adv Drug Delivery Rev 64 suppl, 49–60 (2012)
17. TR Hoare and DS Kohane, Hydrogels in drug delivery: Progress and challenges, Polymer 49(8), 1993–2007 (2008)
18. YH Bae, T Okano and SW Kim, Insulin permeation through thermo-sensitive hydrogels, J Controlled Release 9(3) 271-279 (1989)
19. V Kozlovskaya, E Kharlampieva, I Erelb and SA Sukhishvili, Multilayer-derived, ultrathin, stimuli-responsive hydrogels, Soft Matter 5 4077-4087 (2009)
20. P Gupta, K Vermani and S Garg, Hydrogels: From controlled release to pH-responsive drug delivery, Drug Discovery Today 7(10) 569-579 (2002)
21. TG Park and AS Hoffman, Synthesis and characterization of pH- and/or temperature-sensitive hydrogels, J Appl Polymer Sci 46(4) 659-671 (1992)
22. MS Rao and BC Dave, “Smart” glasses: Molecular programming of rapid dynamic responses in organosilica sol–gels, Adv Mater 14(6) 443–447 (2002)
23. MS Rao and BC Dave, Thermoresponsive glasses: Temperature-controlled rapid swelling and deswelling of silica-based sol–gels, Adv Mater 13(4) 274–276 (2001)
24. CM Burkett, LA Underwood, RS Volzer, JA Baughman and PL Edmiston, Organic-inorganic hybrid materials that rapidly swell in non-polar liquids: Nanoscale morphology and swelling mechanism, Chemistry of Materials 20(4) 1312-1321 (2008)
25. M Gallagher, CJ Wysocki, JJ Leyden, AI Spielman, X Sun and G Preti, Analyses of volatile organic compounds from human skin, BritJ Dermatology 159(4) 780–791 (2008)