Hand sanitizers reduce the microbial load on hands without the need for soap and water. They are an important tool to help reduce the spread of illness-causing microorganisms and are useful as an adjunct to hand-washing. In settings such as health care environments, alcohol-based hand sanitizers are the preferred and recommended method of hand hygiene when the hands are not visibly soiled.1 In addition, the use of alcohol-based hand sanitizers has become an international standard for proper hand hygiene and is strongly endorsed by the Centers for Disease Control (CDC), the Association for Professionals in Infection Control and Epidemiology (APIC), the Society for Healthcare Epidemiology of America (SHEA), the Canadian Public Health Agency, the World Health Organization (WHO), and others.
The use of hand sanitizers is expanding worldwide, in part as a result of efforts by the WHO in its Patient Safety Initiative,2 which now covers more than 85% of the world’s population.3 In fact, in 2007, USA Today included one hand sanitizera in its list of 25 inventions that have changed modern life during the past 25 years, along with the cell phone, laptop computer and the iPodb.4
In the United States, hand sanitizers fall under the purview of the US Food and Drug Administration (FDA) and are regulated as drugs. Current regulations allow hand sanitizer manufacturers to make data-supported antibacterial- related claims such as “kills 99.99% of germs.” Additional claims include, “reduces bacteria that potentially can cause disease” and “decreases bacteria on the skin.”5
The vast majority of hand sanitizers contain short chain alcohols—i.e., ethanol, 2-propanol,1-propanol or combinations thereof—as the active ingredient. In the United States, most hand sanitizers are ethanol-based; however, alternate antimicrobials are found on the market. Following is an overview of the various actives used in hand sanitizers, including their benefits and limitations.
Hand Sanitizer Actives
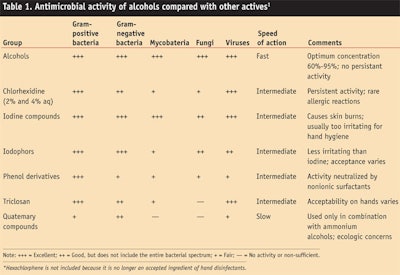
Alcohols: While several types of actives are employed within the hand sanitizer category, alcohols are recognized internationally and identified in various standards and guidelines as being the most effective. The US CDC also lists hand hygiene as the number one preventive measure against the spread of health care acquired infections (HAI) and, as noted, recommends alcohol-based hand sanitizers for hands that are not visibly soiled.1 These and other global endorsements are based on the superior broad-spectrum anti-microbial activity of alcohols, compared with other actives (see Table 1).
Their fast-acting characteristics and rapid evaporation rate also increase productivity and consumer compliance to hand hygiene. In addition, ethanol is the only active generally recognized as safe and effective (GRASE) by the FDA; alcohol-based hand sanitizers have a low irritation potential and are not associated with resistance.
Alcohols have been used as topical antiseptics for centuries and have been suggested for hand disinfection since the late 19th century6 since they kill microorganisms by a very non-specific denaturation of proteins.7 Ethanol in particular has shown outstanding in vitro biocidal activity against vegetative Gram-positive and Gram-negative bacteria, enveloped viruses and a variety of fungi; however, it is ineffective against bacterial spores and has variable activity against enveloped viruses.8
In vivo hand washing tests have shown ethanol (60–70%) and alcohol-based hand sanitizers to be highly effective at reducing the number of viable bacteria9 and, according to the CDC Hand Hygiene Guidelines, to provide consistently better antibacterial activity than standard hand washing with soap.1 Alcohols have also shown greater antiviral activity in vivo than standard hand washing with soap and water.9
While the established efficacy of alcohol and alcohol-based hand sanitizers using laboratory in vitro and in vivo test methods is important, it is also necessary to establish that such products prevent the transmission of disease, which several health care facilities have.10–12 In addition, studies conducted in institutional and commercial settings demonstrated decreased absenteeism and/or infection rates associated with the use of alcohol-based hand sanitizers in place of standard hand washing.13–15 The use of alcohol-based hand sanitizers has also been shown to increase compliance with hand hygiene practices in health care settings.16–18
Quaternary ammonium compounds: Although quaternary ammonium compounds (QACs) are listed as Category III (insufficient data) actives in the 1994 tentative final monograph (TFM),19 several marketed hand sanitizers contain either benzalkonium chloride or benzethonium chloride as the active ingredients.5 QAC-based hand sanitizers often are positioned as non-flammable alternatives to alcohol-based hand sanitizers, or as alternatives where accidental or intentional consumption is a potential concern. While these are positive attributes, the efficacy profile of QACs and their potential for resistance and skin irritation or sensitization (allergy) are significant risks that warrant further scientific assessment.20
The antimicrobial activity of QACs appears to be attributable to their adsorption to the cytoplasmic membrane, with subsequent leakage of low molecular weight cytoplasmic constituents.21 Quats are primarily bacteriostatic and fungistatic, although they are microbiocidal against some organisms at high concentrations.22 They are more active against Gram-positive bacteria than Gram-negative bacilli, they have relatively weak activity against mycobacteria and fungi, and they are somewhat active against lipophilic (enveloped) viruses.
Since quats target microbial membranes, they possess no activity against non-lipophilic (non-enveloped) viruses. Their antimicrobial activity is adversely affected by the presence of organic materials, and they may be neutralized by anionic or nonionic surfactants, hard water, proteins and other moieties.
Published reports on the efficacy of such products are scarce; however, in one laboratory-based study, an alcohol-free hand sanitizer product containing 0.13% benzalkonium chloride was shown to be efficacious in reducing microbial counts on the hands of volunteers.23 Another report demonstrated a 41.9% drop in elementary school absenteeism when students were instructed to use a benzalkonium chloride-based hand sanitizer after entering the classroom, before eating lunch or snacks, after sneezing or coughing, and after using the restroom.24 A clinical study performed among surgical intensive care unit health care workers found that cleaning the hands with antimicrobial wipes containing a quaternary ammonium compound was about as effective as soap and water hand washing, and that both were significantly less effective than alcohol-based hand rubs.25
As noted, antimicrobial resistance to QACs and the potential for the compounds to select or promote antibiotic resistance is a concern.26 Several types of bacteria, including Staphylococcus aureus, can carry genes designated as QAC genes that are encoded to extrude QACs and other noxious compounds from the cell27,28 and in some cases, these genes can be genetically linked to antibiotic resistance genes on transferable plasmids. Although a direct correlation has never been established, studies have demonstrated an association between increased QAC resistance and antibiotic resistance. In fact, recent research has demonstrated higher frequencies of QAC-resistant microorganisms in environments of higher QAC use.
Studies performed by the authors found that several marketed QAC-based hand sanitizers demonstrated poor biocidal activity against methicillin resistant S. Aureus (MRSA) isolates. The activity was found to vary not only from product to product but was highly dependent upon the particular MRSA isolate tested.29 Although these tests did not identify the mechanism of resistance, they point to a weakness in QACs to act as effective hand sanitizers.
Triclosan: Triclosan (2,4,4’–trichloro-2’-hydroxydiphenyl ether) is a nonionic, chlorinated phenolic compound used widely as an ingredient in antimicrobial soaps for use by health care workers and consumers. It also is used in toothpastes, mouthwashes and other personal care products, as well as materials such as plastics for antimicrobial coatings. Recently it has been used as an active ingredient in some leave-on topical antiseptics.
At biocidal concentrations (0.2–2.0%), triclosan kills microorganisms by damaging cell membranes.30 At lower concentrations, it is bacteriostatic and has been shown to target enoyl reductase, which is required by living organisms for fatty acid biosynthesis.31
Triclosan has a broad range of anti-microbial activity but demonstrates weak activity against certain Gram-negative bacteria, particularly P. aeruginosa.22
From a formulating standpoint, the material is poorly water-soluble and tends to partition into micelles with surfactants. Therefore, it can be difficult to formulate with in a manner that maintains its bactericidal activity. For this reason, the antibacterial activity of triclosan-containing products is highly formulation dependent. While much data exists on the in vivo efficacy of triclosan-containing hand washes, little if any published data is available to support the use of triclosan in leave-on products.
Triclosan recently has been scrutinized due to concerns for environmental accumulation and potential health risks associated with its use—although there is debate regarding the validity of these concerns.26 Triclosan is also a Category III ingredient in the FDA’s TFM yet remains a popular ingredient for antimicrobial hand washes. However, a recent warning letter from the FDA to a manufacturer cautioned that triclosan is not approved for use in no-rinse situations and may thus limit its future use in hand sanitizers in the United States.
Natural antimicrobials: Along with the trend for more environmentally conscious products, demand has grown for natural antimicrobials. Several existing hand sanitizers are based on natural ingredients such as thymol; however, no data has been published to date on their in vivo efficacy. And since natural ingredients are not addressed in the TFM, these products cannot legally be marketed in the United States without an approved new drug application (NDA).
Hand Sanitizer Forms
Alcohol-based hand sanitizers originally were developed as thin liquid rinses and in this form, while they provide antimicrobial efficacy, they are messy and can cause skin irritation via stripping of skin lipids, depending on their method and frequency of use. While some cultural regions such as parts of Europe tolerate this form, others such as the United States require a more practical and frequently usable solution. As such, alcohol-based gels were developed to provide a less messy alternative. Gels also have become a format for the delivery of emollients and moisturizers, which was a breakthrough in skin care performance and aesthetics—critical components of usage and compliance.1
Foaming alcohol delivered via an aerosol can has existed for years but their costs have been a major market acceptance barrier. Recent formulation work has thus focused on non-aerosol foaming alcohol technologies. Alcohol-based foam hand sanitizers have rapidly become a leading product within health care markets due to their lower risk for messiness and floor staining.
Even newer forms of hand sanitizers include sprays and wipes. Spray products can be designed as atomized dispensing systems resulting in a fine mist similar to traditional perfumes, as well as a liquid spray similar to traditional hard surface liquid sprays. Sprays are viewed as providing better coverage and therefore better efficacy, although there is no strong evidence to support this belief. Wipes, on the other hand, are liquid products delivered from a disposable clothlike substrate. Wipes are believed to provide waterless cleaning and hand sanitizing, although the clinical evidence is very limited.
Limits and Opportunities
Despite the clear benefits of alcohol-based hand sanitizers, they do have limitations. Most notably, hand sanitizers do not remove dirt or other soil from the hands and they do not kill bacterial endospores such as anthrax or Clostridium difficile.7, 32, 33 Many patented technologies exist that claim to provide a means of waterless cleaning with hand sanitizing—e.g., polymer-based powders that crumble off the hands after use—but none have been reduced to marketable, consumer accepted products. Nevertheless, it has been shown that antimicrobial efficacy can be achieved even in the presence of soils such as blood,34 and some could argue that sterile, dirty hands are better than visibly clean, micro-contaminated hands.
Most current alcohol-based hand sanitizers claim to contain moisturizers and evidence suggests that in most applications, these products have no net effect on skin health,35 despite erroneous public perceptions that they are drying the skin.
It has been shown that the use of hand sanitizers in place of frequent hand washing actually results in improved skin condition for health care workers36 and recent advancements have made it possible to include skin care ingredients that improve the condition of health care workers.37 However, a hand sanitizer that delivers broad-spectrum antimicrobial efficacy while truly providing skin care performance and eliminating the need for hand lotion remains a consumer need in some markets.
The more recent focus of hand sanitizer development has been to improve activity against harder-to-kill, non-enveloped viruses. Several publications have described alcohol-based hand sanitizers with improved efficacy against non-enveloped viruses.38–40 These sanitizers are based on alcohol and include ingredients to potentiate the activity of alcohol against specific viruses. For example, Kramer et al. described a system based on a combination of ethanol, 1-propanol and diols, and phosphoric acid that improved the activity against several non-enveloped viruses.38 In addition, Macinga et al. disclosed a potentiated system based on ethanol that exhibited increased activity against several non-enveloped viruses.39
Conclusions
In conclusion, hand sanitizing is now a proven public health benefit that is growing globally. Alcohol is the most tested and proven and thus most commonly used active ingredient for hand sanitizing. When formulated and tested effectively, hand sanitizers deliver antimicrobial efficacy especially against bacteria and viruses, as well as non-irritating and non-sensitizing skin care. In addition, they are aesthetically acceptable. When one couples these performance attributes with the intrinsic benefits of cost, portability and convenience, the result is the successful, relatively new category of hand sanitizing.
References
1. JM Boyce and D Pittet, Guideline for hand hygiene in Health-care Settings CDC Morbidity and Mortality Weekly Report 52 (RR-16) (2002)
2. World Health Organization, WHO guidelines on hand hygiene in healthcare WHO, Geneva, 26–27 (2006)
3. D Pittet, Plenary presentation, 2008 Annual Meeting of the Society of Hospital Epidemiologists, Orlando, FL USA (2008)
4. B Acohido, J Hopkins, J Graham and M Kessler, 25 years of ‘eureka’ moments, USA Today (May 2007)
5. The US Food and Drug Administration, Tentative final monograph for health-care antiseptic drug products, proposed rule, Federal Register 59(116) 31402–31452 (1994)
6. P Fürbringer, Zur desinfection der hände des arztes, Dtsch Med Ws 48 985–987 (1888)
7. Y Ali, MJ Dolan, EJ Fendler and EL Larson, Alcohols disinfection, sterilization and preservation, etd block, Lippincott Williams and Wilkins: Philadelphia, USA, 12 (2001)
8. G Kampf, M Höfer and C Wendt, Efficacy of hand disinfectants against vancomycin-resistant enterococci in-vitro, J Hosp Infect 42(2) 143–50 (1999)
9. ML Rotter, Handbook of Disinfectants and Antiseptics, Marcel Dekker Inc.: New York, 177–233 (1996)
10. EJ Fendler, Y Ali, BS Hammond, MK Lyons, MB Kelley and NA Vowell, The impact of alcohol hand sanitizer use on infection rates in an extended care facility, Am J Infect Control 30 226–233 (2002)
11. J Hilburn, BS Hammond, EJ Fendler and PA Groziak, Use of alcohol hand sanitizer as an infection control strategy in an acute care facility, Am J Infect Control 31 109–116 (2003)
12. WE Trick et al, Impact of ring wearing on hand contamination and comparison of hand hygiene agents in a hospital, Clinical Infectious Disease 36 1383–1390 (2003)
13. B Hammond, Y Ali, E Fendler, M Dolan and S Donovan, Effect of hand sanitizer use on elementary school absenteeism, Am J Infect Control, Oct 28(5) 340–6 (2000)
14. M Guinan, M McGuckin and Y Ali, The effect of a comprehensive hand washing program on absenteeism in elementary schools, Am J Infect Control 30 217–220 (2002)
15. C White et al, The effect of hand hygiene on illness rate among students in university residence halls, Am J Infect Control 31 364–370 (2003)
16. S Harbarth et al, Interventional study to evaluate the impact of an alcohol-based hand gel in improving hand hygiene compliance, Pediatr Infect Dis J 21 489–95 (2002)
17. S Hugonnet, TV Perneger and D Pittet, Alcohol-based hand rub improves compliance with hand hygiene in intensive care units, Arch Intern Med 162(9) (2002)
18. D Pittet et al, Effectiveness of a hospital-wide program to improve compliance with hand hygiene, Infection Control Programme Lancet 356 1307–12 (2000)
19. US Food and Drug Administration Web site, www.fda.gov/OHRMS/DOCKETS/ac/05/briefing/2005-4184B1_01_16-FDA-TAB15.pdf (Accessed Oct 21, 2009)
20. C Fricker and J Arbogast, A technical and literature assessment of skin compatibility of benzalkonium chloride and benzethonium chloride, accepted for poster presentation at Occupational and Environmental Exposure of Skin to Chemicals (OEESC) International Conference (2009)
21. JJ Merianos, Disinfection, Sterilization and Preservation, 4th edn, Lea and Febiger: Philadelphia, USA, 225–255 (1991)
22. M Rotter, Hand Hospital Epidemiology and Infection Control, 2nd ed, Lippincott Williams and Wilkins, Philadelphia USA, 1339–1355 (1999)
23. DL Dyer et al, Testing a new alcohol-free hand sanitizer to combat infection, Assoc of Peri Operative Registered Nurses J 68:239-251 (1998)
24. DL Dyer, A Shinger and Shinder, Alcohol-free instant hand sanitizer reduces elementary school illness absenteeism, Fam Med 32 633–638 (2000)
25. RA Hayes et al, Comparison of three hand hygiene methods in a surgical intensive care unit, American Society for Microbiology, Proceedings of the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago (2001)
26. SCENIHR, Assessment of the antibiotic resistance effects of biocides, http://eceuropaeu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_021.pdf (2009)
27. G McDonnell and AD Russell, Antiseptics and disinfectants: Activity, action and resistance, Clin Microbiol Rev 12 147–179 (1999)
28. AD Russell, Plasmids and bacterial resistance to biocides, J Appl Microbiol 82 155–165 (1997)
29. SL Edmonds, C Bondi, CA Zapka, D Wieland, DR Macinga and JW Arbogast, In vitro susceptibility of hospital-acquired, community-acquired, and laboratory MRSA strains to topical antiseptics (oral presentation), American Public Health Association Annual Conference, San Diego USA (2008)
30. RD Jones, HB Jampani, JL Newman and AS Lee, Triclosan: A review of effectiveness and safety in health care settings, Am J Infect Control 28 184–96 (2000)
31. RJ Heath, YT Yu, MA Shapiro and E Olson, Broad spectrum antimicrobial biocides target the FabI component of fatty acid synthesis, J Biol Chem, Nov 13, 273(46) 30316–20 (1998)
32. B Setlow et al, Mechanism of killing spores of Bacillus subtilis by acid, alkali and ethanol, J of Applied Microbiology, 92 362–375 (2002)
33. M Wult, I Odenholt and Walder, Activity of three disinfectants and acidified nitrite against Clostridium difficile spores, Infection Control and Hospital Epidemiology 24 765–768 (2003)
34. L Bobo and EL Larson, Effective hand de-germing in the presence of blood, J of Emergency Medicine 10 7–11 (1992)
35. E Fendler, B Hammond, M Dolan and R Williams, Treatment of irritant contact dermatitis in the health care occupations, American Academy of Dermatology Annual Meeting, Mar 19–24, New Orleans USA (1999)
36. JM Boyce, S Kelliher and N Vallande, Skin irritation and dryness associated with two hand hygiene regimens: Soap-and-water hand washing versus hand antisepsis with an alcoholic hand gel, Infect Control Hosp Epidemiol 21 442–448 (2000)
37. C Bondi, KA Dobos, T Cartner, D Salisbury, DL Miller and JW Arbogast, Moisturization performance of a novel, foaming instant hand sanitizer (IHS) containing a synergistic skin moisturizing blend, Dermal Clinical Evaluation Society Spring Meeting and Poster Session (2008)
38. A Kramer et al, Virucidal activity of a new hand disinfectant with reduced ethanol content: Comparison with other alcohol-based formulations, J Hosp Infect 62 98–106 (2006)
39. DR Macinga, SA Sattar, LA Jaykus and J W Arbogast, Improved inactivation of nonenveloped enteric viruses and their surrogates by a novel alcohol-based hand sanitizer, Appl Environ Microbiol 74 5047–5052 (2008)
40. SA Sattar, M Abebe, AJ Bueti, H Jampani, J Newman and S Hua, Activity of an alcohol based hand gel against human adeno-, rhino- and rotaviruses using the fingerpad method, Infect Control Hosp Epidemiol 21 516–519 (2000)