Skin appearance and functionality are affected by a complex combination of factors including both genetic, i.e. intrinsic, and actinic, i.e. extrinsic or environmental. Indeed, genetic and actinic factors act together to modulate the expression of key genes involved in skin homeostasis. Intrinsic aging is genetically regulated and follows a chronological clock inside of cells, while environmental factors such as UV exposure, humidity and air pollutants are responsible for actinic aging. Together, genetic and actinic aging target important metabolic pathways in skin cells that trigger the signs of aging such as skin roughness and wrinkling.
At a molecular level, it has been demonstrated that collagen synthesis is reduced in aged skin cells and in cells damaged by UV radiation.1 Similarly, the expression of various matrix metalloproteases (MMPs), which cause the degradation of collagen fibers and other skin fibrillar components, is upregulated in aged cells as well as in cells exposed to UV, infrared radiation or excessive heat.2, 3 Furthermore, reactive oxygen species are molecular agents implicated in the deleterious effects of both intrinsic and extrinsic aging.1, 4
The skin barrier also plays important roles in skin’s functioning. For instance, it prevents excessive water loss and protects against physical trauma, foreign particles such as allergens, bacteria, toxic chemicals, and also radiation. The barrier function of the skin mainly resides in the stratum corneum (SC) layer of the epidermis, where differentiated keratinocytes are embedded within a lipid matrix. With age, the ability of the barrier to repair damage from physical insults decreases.5
In an effort to develop an active to improve skin barrier repair and hydration, increase elasticity, and reduce the appearance of wrinkles, the algae extract Saccharina longicruris (S. longicruris) was investigated since algae is known for its survival abilities. Specifically, it develops thickened segments to adapt to water movements and stores compounds to survive harsh environments. Thus, to gain insight into the functionality of S. longicruris extract, the author examined its efficacy to promote keratinocyte differentiation and reverse the gene expression level of senescent human fibroblasts, described here.
S. longicruris Properties and Formulation
S. longicruris is a laminaria algae manually harvested during fall in the cold waters of the North Atlantic before the area is covered by ice. The plant grows up to 1 cm/day during the summer period and accumulates nutrients and polysaccharides (laminarans) to survive throughout the winter season when water temperatures are as low as 1°C. It uses these nutrients and polysaccharides to restart the growth phase the following spring. When the algae is exposed to such extreme conditions, the activation of selective metabolic pathways is triggered as well as the production of specific compounds within the algae tissues. Interestingly, it has been shown that some plants have the ability to produce protective compounds when exposed to low temperatures.6
An aqueous extract of S. longicrurisa was therefore developed and its efficacy examined to determine its effects on the cellular metabolic deregulations and in situ cutaneous surface topography modifications that result in skin aging. The clear, yellowish, odorless extract typically comprises laminarans and fucoidans in roughly equal proportions, and molecular sieving was used to demineralize the extract and select a polysaccharide fraction. The extract was then formulated in an aqueous base containing 70% glycerin to create a preservative-free system that is soluble in all water-based environments
Materials and Methods
Specific characteristics of S. longicruris such as physical protection against water movements and the accumulation of potential protection compounds led to research in its ability to promote epidermal keratinocyte differentiation and to regenerate the metabolic activity of senescent skin fibroblasts. Clinical benefits for the skin barrier function and signs of aging were investigated.
Keratinocyte differentiation: Transglutaminases are enzymes activated in the outermost layer of the epidermis to introduce the cross-linking of proteins of the cornified envelope, thus providing stability and mechanical resistance to the skin barrier function. Therefore, the enzymatic activity of transglutaminase K (TGK) is considered a marker for keratinocyte terminal differentiation.7 Normal human epidermal keratinocytes (NHEK) were incubated for 96 hr in 0.75% S. longicruris or calcium chloride as a positive control. At the end of the incubation, TGK was extracted from NHEK cell membranes, and TGK enzymatic activity was measured using the appropriate kitb according to the manufacturer’s instructions.
Gene expression: Normal human dermal fibroblasts (NHDF) were obtained after the eighth passage (P8) or the fourteenth in vitro culture passage (P14). P8 cells were referred to as young or normal fibroblasts, while P14 cells were referred to as aged or senescent fibroblasts. Fibroblasts were incubated in the presence or absence of 0.75% S. longicruris for a period of 24 hr. At the end of the incubation, cells were washed, lysed and stored at -80°C. Analysis of gene expression was performed using standard minichips with dedicated cDNAc.
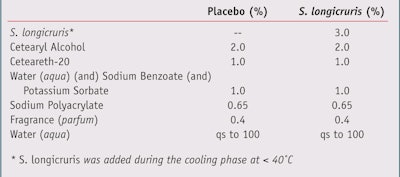
Clinical trial and dermatological evaluations: Twenty healthy volunteers ages 35—65 with mixed skin types were enrolled in a trial where a single-blind, split-face, randomized protocol was used to evaluate the efficacy of a placebo formulation against a formulation containing 3% S. longicruris (see Table 1). The trial was held in the summer (May to July) in Europe. The volunteers were given 120 g of the formulations (2 x 60 g jars) for the duration of the trial, applying the product at home for the period of 60 days.
Skin hydrationd, transepidermal water loss (TEWL)e and skin elasticityf were measured at baseline prior to the application of the formulations and after 15, 30 and 60 days of twice daily applications of a placebo and a formulation containing S. longicruris. At the same experimental time points, skin surface smoothness, skin wrinkle density and wrinkle volume were assessed in situ by skin surface optical digitalizationg.8 Statistical analyses were performed using the bilateral t-test for paired and unpaired data.
Further, a dermatologist evaluated skin smoothness, skin compactness and wrinkle appearance at baseline and each experimental time point. Skin smoothness was evaluated based on the following criteria and corresponding scores: skin not smooth (1), insufficient smoothness (2), skin smooth (3) and skin very smooth (4). Evaluation criteria and corresponding scores for skin compactness were: skin not compact (1), insufficient compactness (2), skin compact (3) and skin very compact (4). Wrinkle density was assessed based on seven progressive criteria and corresponding scores varying from: no visible wrinkles (0), to deep wrinkles and furrows (3). Results for wrinkle appearance were reported as the degree of improvement with corresponding scores for each time point compared to baseline as: no variation (1), slight improvement (2), moderate improvement (3) and remarkable improvement (4). Statistical analyses were performed using the Wilcoxon-signed rank test for non-parametric data.
Results and Discussion
The efficacy of a S. longicruris extract was examined in a variety of tests in order to link S. longicruris behaviors and acclimation to environmental stress.
Barrier function and hydration: As noted, S. longicruris has developed mechanisms to protect itself from harsh environments; specifically, portions of the laminae exposed to high water movements (waves) have a reduced metabolic activity and will adopt a narrow and thickened “differentiated” morphology for better resistance.9 As the cells of the SC have a role in protecting inner skin layers from environmental insult, the ability of the S. longicruris extract to stimulate keratinocyte differentiation was investigated first. Results indicated that when added to unstimulated keratinocytes, S. longicruris increased the activation of transglutaminase by 349%, as shown in Figure 1. It was therefore concluded that the ability of S. longicruris to activate transglutaminase in keratinocytes is likely to improve the barrier function of the skin.
Further, the pro-barrier effect of S. longicruris was clinically confirmed by TEWL measurements. TEWL is correlated with the skin barrier status10 and topical applications of a formulation containing 3% S. longicruris significantly reduced TEWL. An effect was observed on day 15 of the clinical trial (-8.4%, p < 0.0001) that increased up to day 60 (-13.4%, p < 0.0001), as illustrated in Figure 2.
In relation to skin barrier function, Figure 3 shows the positive action of S. longicruris on the skin barrier function by improving the skin hydration level. The application of the formulation containing S. longicruris led to an incremental increase in corneometry measurements with a 24% augmentation after 60 days. Additionally, a significant 15.3% increase in hydration was obtained on day 15.
Anti-aging efficacy and clinical benefits: To gain more detailed information about the cutaneous biological potential of S. longicruris extract, gene expression analyses using dedicated cDNA were performed. Expression of selected genes was assessed in young (P8) and senescent (P14) fibroblasts. The potential revitalization of senescent gene expression of S. longicruris was verified by incubating P14 in the presence of the algae extract.
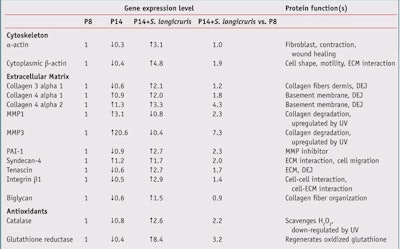
The effect of cellular aging on the gene expression level and the anti-aging action of S. longicruris are reported in Table 2, where studied genes are grouped according to their cellular functions. In Table 2,the expression level for each gene was arbitrarily set to a value of 1 in young fibroblasts (P8). The effect of in vitro aging on gene expression level is shown in P14. Any reduction in gene expression is shown with a down arrow, and any increase in gene expression is represented with an up arrow.
The effect of S. longicruris on senescent fibroblasts is shown in P14 + S. longicruris. For example, the expression of α-actin in senescent cells was reduced to 30% of the control value (expression level in P8 cells). As a major constituent of the cytoskeleton (internal cell scaffolding), α-actin is actively involved in cell shape, function, motility and signaling. The addition of S. longicruris to senescent cells (P14) increased the gene expression of α-actin by 3.1-fold. Therefore, it can be concluded that the gene expression level of α-actin in senescent fibroblasts was restored to a level observed in young cells (P14 + S. longicruris vs P8). In the case of α-actin gene expression, S. longicruris produced a complete rejuvenating action. In addition, the extracellular matrix (ECM) restructuring action of S. longicruris was supported by its effect on the gene expression of collagens, MMPs and plasminogen activator inhibitor-1 (PAI-1), an endogenous MMP inhibitor.
Collagen: A reduced expression of specific subunits of collagen 3 and 4 was observed in senescent fibroblasts. The addition of S. longicruris restored the collagen gene expression level close to or above what was observed in young cells. Similar results were also noted for proteins involved in the ECM tridimensional organization like syndecan-4, tenascin, integrin β1 and biglycan.
MMPs: The expression of MMP-1 and MMP-3, enzymes that cause the degradation of structural components of the ECM, was clearly increased in senescent fibroblasts when compared to their young counterparts. An age-related increase in the expression and activity of MMPs in skin cells has been also demonstrated elsewhere.11 The presence of S. logicruris diminished the upregulation in MMP-1 and MMP-3 gene expression as well.
PAI-1: PAI-1 is an inhibitor of the MMPs that break down collagen and elastin fibers. An increase in PAI-1 expression would help reduce the catabolic activity of MMPs in destroying components of the ECM. Interestingly, the expression of the endogenous MMP inhibitor PAI-1 was boosted by S. longicruris; its increase in PAI-1 expression points toward a reduction of the catabolic process of the ECM components as observed during intrinsic and actinic aging.
The increase in PAI-1 expression and a down-regulation of MMPs demonstrates that S. longicruris imparts an anti-aging action and/or directly stimulates genes involved in the integrity of the ECM. Furthermore, the expression of the cytoplasmic β-actin, which is involved in cell motility and cell interaction with the ECM, in senescent cells was also rescued by the addition of S. longicruris.
Antioxidant enzymes: The expression of catalase and glutathione reductase, two protective antioxidant enzymes, is negatively affected by cellular aging. A reduction in skin catalase activity has also been reported after exposure to UV radiation.12 The reduction in expression of catalase and glutathione reductase would diminish the ability of skin to eliminate reactive oxygen species. However, S. longicruris not only normalized but enhanced the expression of catalase and glutathione reductase when added to senescent fibroblasts.
S. Longicruris and Skin Surface Topography
Based on the gene expression profile comparing young and senescent fibroblasts and the effects of S. longicruris on agingrelated gene expression, the clinical efficacy of S. longicruris to reduce the appearance of aging was measured. For these measurements, a high resolution UVA video camera was usedg in situ for optical skin topography measurements. Image digitalizations of the skin topography before and after treatment with S. longicruris are shown in Figure 4.
In Figure 4, skin surface smoothing and a reduction in wrinkle profilometry were observed (designated by white arrows). The formulation containing S. longicruris significantly increased the skin surface smoothness parameter starting on day 15 (+12.2%; p < 0.0001, compared to baseline) reaching +17.8% (p < 0.0001, compared to baseline) at day 60, as shown in Table 3. Skin surface smoothness was calculated by the mean wrinkle width and depth.
Skin wrinkling density, calculated from the average number and width of horizontal and vertical wrinkles, also was significantly diminished from day 15 to day 60 (from -4.9%: p < 0.005, to -11.5%; p < 0.0001, respectively, compared with the baseline). In addition, wrinkle volume was significantly reduced by 10.1%, 1.2% and 15.7% on day 15, 30 and 60, respectively (p < 0.0001, compared with the baseline). It was therefore concluded that topical applications of a S. longicruris formulation significantly improved the skin surface profilometry (surface smoothness, wrinkle density and volume) as quantified by analyses.
Skin elasticity: The cell culture and clinical trial results obtained with the use of a formulation containing S. longicruris were in line with improvements of the ECM integrity and skin water reservoir capability. And since skin visco-elastic properties rely primarily on these parameters, the efficacy of S. longicruris using cutometry measurements was been assessed. Indeed, the application of a formulation containing S. longicruris augmented skin elasticity at all time points of the trial, as shown in Figure 5. An 11.1% improvement of skin elasticity was observed at day 15 (p < 0.0001, compared with the baseline); a 12.7% improvement was observed at day 30 (p < 0.0001, compared with the baseline); and a 13.0% improvement was observed on day 60 (p < 0.0001, compared with the baseline). Interestingly, although the action of S. longicruris progressively increased throughout the trial, a near-maximal effect was obtained at day 15 (p > 0.05 for day 15, versus day 30 or day 60).
Dermatologist evaluations: Visual improvements of skin smoothness, compactness and wrinkle appearance were further evaluated by a dermatologist at all time points of the trial based on the criteria described in the Materials and Methods section. As shown in Figure 6, dermatological evaluations revealed that these parameters were improved by 80%, 55% and 55%, respectively, for subjects that had applied the formulation containing S. longicruris for 60 days. These results are in agreement with the visioscan and cutometry measurements obtained in the same clinical trial. It is noteworthy that none of the subjects who had applied the placebo demonstrated an improvement in the parameter of wrinkle appearance at any time point.
Conclusions
Skin has to cope with a double aging threat; one intrinsic, or genetically driven, and one actinic where skin is prone to environmental stressors. The structural and functional integrity of the dermis and the epidermis become progressively diminished upon intrinsic aging, and more acutely by actinic aging. Increased MMP activity and the ensuing breakdown of fibrillar components of the ECM, compromised skin barrier and the weakening of the skin antioxidant defense network are examples of the consequences of aging-linked skin alterations. On a more aesthetic aspect, skin dryness, skin sagging and the appearance of wrinkles visually translate the ongoing aging process.
The efficacy of S. longicruris in cell-based assays and clinical trials demonstrated the material’s anti-aging effect. For example, the results reported in the cDNA experiments have shown that S. longicruris has the ability to restore gene expression observed in senescent skin cells to levels close to those detected in young ones. In addition, clinical benefits of S. longicruris were shown for skin barrier function, skin hydration, skin elasticity and skin surface topography including a signifi cant reduction in the appearance of wrinkles. A parallel improvement in skin hydration and parameters such as elasticity and surface topography supports the interdependence between the barrier function and skin mechanical properties as reported by Pedersen and Jemec.13
The in vitro results demonstrated here suggest that S. longicruris acts on both keratinocytes and fibroblasts. While it is difficult to pinpoint a specific mechanism(s), as the S. longicruris extract tested is not chemically defined apart from the content in polysaccharides, different scenarios could be proposed to explain the results obtained. On one hand, keratinocytes activated upon the application of the polysaccharide-containing S. longicruris extract could, through a cascade signaling pathway, affect the metabolic activity of fibroblasts. Indeed, it is well-established that there is a signaling cross-talk between keratinocytes and fibroblasts upon exposure to a stress,14 or during the process of wound healing.15
On the other hand, the topically applied S. longicruris polysaccharides could attain fibroblasts, exerting a direct biological action. In support of that scenario, long fragments (~400 kDa) of hyaluronan were shown to penetrate the epidermis when applied topically.16 Furthermore, a reduction of wrinkles associated with an increase in collagen and elastic fibers were also reported for topically applied fragments of hyaluronan (50-400 kDa).17 Further studies are required to unravel the precise mechanisms of action of S. longicruris. However, the present work supports the positioning of S. longicruris as a potent anti-aging technology.
References
Send e-mail to [email protected].
1. TM Callaghan and KP Wilhelm, A review of ageing and an examination of clinical methods in the assessment of ageing skin. Part I: Cellular and molecular perspectives of skin ageing, Int J Cosmet Sci 30 5 313–322 (2008)
2. T Quan, Z Qin, W Xia, Y Shao, JJ Voorhees and GJ Fisher, Matrix-degrading metalloproteinases in photoaging, J Investig Dermatol Symp Proc, 14 1 20–24 (2009)
3. S Cho, MH Shin, YK Kim, J-E Seo, YM Lee, C-H Park and JH Chung, Effects of infrared radiation and heat on human skin aging in vivo, J Investig Dermatol Symp Proc 14 1 15–19 (2009)
4. DR Bickers and M Athar, Oxidative stress in the pathogenesis of skin disease, J Invest Dermatol 126 12 2565–2575 (2006)
5. R Ghadially, BE Brown, SM Sequeira-Martin, KR Feingold and PM Elias, The aged epidermal permeability barrier. Structural, functional, and lipid biochemical abnormalities in humans and a senescent murine model, J Clin Invest 95 5 2281–2290 (1995)
6. W Bilger, M Rolland and L Nybakken, UV screening in higher plants induced by low temperature in the absence of UV-B radiation, Photochem Photobiol Sci 6 2 190–195 (2007)
7. K Hitomi, Transglutaminases in skin epidermis, Eur J Dermatol 15 5 313–319 (2005)
8. H Tronnier, M Wiebusch, U Heinrich and R Stute, Surface evaluation of living skin, Adv Exp Med Biol 455, 507–516 (1999)
9. VA Gerard and KH Mann, Growth and production of Laminaria longicruris (Phaeophyta) populations exposed to different intensities of water movement, J Phycol 15 33–41 (1979)
10. JW Fluhr, KR Feingold, PM Elias, Transepidermal water loss refl ects permeability barrier status: validation in human and rodent in vivo and ex vivo models, Exp Dermatol 15 7 483–492 (2006)
11. GJ Fisher , T Quan , T Purohit , Y Shao , MK Cho , T He , J Varani , S Kang and JJ Voorhees, Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase- 1 in fi broblasts in aged human skin, Am J Pathol 174 1 101–14 (2009)
12. GE Rhie, JY Seo and JH Chung, Modulation of catalase in human skin in vivo by acute and chronic UV radiation, Mol Cells 11 3 399–404 (2001)
13. L Pedersen, GB Jemec, Mechanical properties and barrier function of healthy human skin, Acta Derm Venereol 86 4 308–311 (2006)
14. S Trompezinski, I Pernet, D Schmitt and J Viac, UV radiation and prostaglandin E2 up-regulate vascular endothelial growth factor (VEGF) in cultured human fi broblasts, Inflamm Res 50 8 422–427 (2001)
15. S Werner, T Krieg and H Smola, Keratinocytefibroblast interactions in wound healing, J Invest Dermatol 127 5 998–1008 (2001)
16. TJ Brown, D Alcorn and JR Fraser, Absorption of hyaluronan applied to the surface of intact skin, J Invest Dermatol 113 5 740–746 (1999)
17. G Kaya, C Tran, O Sorg, R Hotz, D Grand, P Carraux, L Didierjean, I Stamenkovi c and JH Saurat, Hyaluronate fragments reverse skin atrophy by a CD44-dependent mechanism, PLoS Med 3 12 e493 (2006)
Lab Practical: S. longicruris
- This extract is a clear, yellowish solution that exhibits no specific odor.
- It typically comprises laminarans and fucoidans in equal amounts.
- Formulated in an aqueous base with 70% glycerin, the extract is soluble in water-based environments. It is also preservative-free.
- The extract should be added during cooling or at the end of the emulsion preparation.