Skin pigmentation is a major concern for most humans. It not only provides information about race, age, gender, social status, culture, habits and level of health, but it also is a major determinant of physical attractiveness. Not long ago, tanned skin was the gold standard in the United States for women and men seeking a better, healthier look. However, both physicians and consumers have since learned that exposure to UV radiation to achieve skin tanning can result in a number of negative effects, specifically more wrinkles, the appearance of skin pigmentation, and an increased risk of skin cancer.
Today’s society has experienced the return of a trend for whiter skin. The phenomenon is spreading worldwide, although for different reasons. In Asia, where its citizens are affected early by uneven skin tone,1 whitening products are popular. These products are used daily by a broad range of consumers to preserve the appearance of healthy skin, especially on the face.2 In countries such as India, there is strong social pressure for paler skin, which is considered more beautiful.3 Lightening products are also gaining popularity in the Western world. Caucasian consumers, however, are more concerned with the appearance of age spots on their hands, décolleté and cheeks. This is a different market, mainly composed of older consumers.
The global skin lightening and whitening market is expected to reach US $10 billion by 2015.4 This demand certainly warrants a better understanding of the skin pigmentation processes, which is described here. Further, it supports the R&D strategy to develop actives that provide skin-whitening effects in personal care products, such as the peptide discussed below.
Melanin and Skin Pigmentation
Skin pigmentation is the result of a mosaic of brown and red pigments on a white background. Although influenced by the underlying reddish tone of microvessels, the main pigment responsible for the color of human skin is melanin.
Melanin is synthesized in melanosomes, which are specialized organelles found in melanocyte cells. Two types of melanin are found: red pigment (pheomelanin) and dark brown pigment (eumelanin). The basal expression, proportion and alignment of both pigments are written within human DNA in a manner highly specific to each individual that determines one’s skin type.5 The blueprint for these pigments can be thought of as the sheet music for a melanogenic symphony. The interpretation of the music by the melanogenic symphony is open to external factors; for example, it may become louder and change tempo under the influence of UV rays, reactive oxygen species (ROS) production, inflammation, hormones, health problems or even the simple passage of time.6
‘Musicians’ in the Melanogenic Symphony
Music would remain silent without musicians, so to understand the melanogenic symphony completely, one must take a closer look at them. Tyrosinase may qualify as the first-chair flutist of a wind band, the leading voice that has the duty of tuning its section of the orchestra. Tyrosinase is a key enzyme in the control of melanogenesis. It sets the tone by transforming tyrosine molecules into L-3,4-dihydroxyphenylalanine (L-DOPA), and with the support of tyrosinase-related proteins (TRP-1 and TRP-2) catalyzing further reactions, eumelanin and pheomelanin can then be produced from L-DOPA.6
Yet despite their clear importance for the musical performance, tyrosinase, TRP-1 and TRP-2 are under the influence of microphthalmia-associated transcription factor (MITF), the orchestra leader. MITF is a transcription factor controlling not only the expression of tyrosinase, TRP-1 and TRP-2, but also the expression of other genes involved in melanosome genesis and transport, making it the most critical transcription factor for melanogenesis.7 MITF is the point of convergence of the constitutive (individual phototype) and the facultative (UV-induced) pathways of melanogenesis (see Figure 1).
MITF integrates signals originating from alpha-melanocyte-stimulating hormone (α-MSH), which is part of the facultative melanogenic pathway. When triggered by external factors such as UV light, α-MSH binds to the MC1-Receptor on melanocytes, initiating a signaling cascade involving the production of cyclic adenosine monophosphate (cAMP) and the activation of protein kinase A (PKA). The production of cAMP and the activation of PKA lead to the activation of the CREB nuclear factor, which binds to MITF and turns it on, increasing the melanogenic melody to forte (loud) and allegro (fast).8
The constitutive pathway has recently been associated with transforming growth factor-β1 (TGF-β1), which acts as a paracrine modulator of pigmentation.10 TGF-β1, bound to its receptor at the melanocyte surface, stimulates a signaling cascade involving RAS activation and extracellular signal-regulated kinases (ERK) phosphorylation. Subsequently, MITF is down-regulated and, through proteasome mediation, is degraded.7 This may allow the melanogenic melody to resume its tempo primo or initial pace, following activation of the facultative melanogenic pathway.
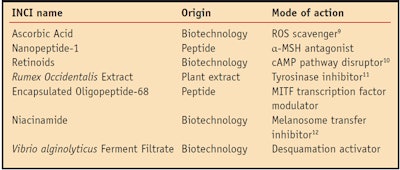
As a whole, skin pigmentation is a complex biochemical and biological process that, like the symphony, mobilizes many performers to control melanosome maturation and transport, melanocyte shape changes (dendricity), and melanosome transfer and uptake by keratinocytes; and ultimately skin desquamation.11 Given the complexity of the pigmentation process, it seems appropriate to target multiple mechanisms to lighten or whiten skin since inhibiting a single performer would not be sufficient for a noticeable decrescendo of the music. Such a strategy would obviously involve various materials and address several processes; some of which are listed in Table 1. In addition, given the importance of MITF, an effective whitening/lightening strategy also should address the performance of the orchestra leader.
Oligopeptide-68 Active
To inhibit both constitutive and facultative melanogenesis, MITF activity must be decreased. Therefore, an active was developed based on oligopeptide-68a, a biomimetic peptide derived from TGF-β since TGF-β1 stimulates a cascade that down-regulates MITF, as noted, thus inhibiting its activity. The biomimetic peptide was encapsulated in a liposome preparation to maximize skin penetration, and in vitro and clinical studies were conducted with the active as a whitening/lightening agent to determine its efficacy.
In vitro Studies Western blot analysis: B16-F10 cells were treated with oligopeptide-68 (0.1 mg/mL) for 30 min. Cells were then lysed and levels of MITF, tyrosinase and phosphorylated ERK1 and ERK2 proteins were detected in lysate using sodium dodecyl sulfate polyacrylamide gel electrophoresis and immunoblotting techniques. Specific antibodies for β-actin (internal standard), tyrosinase, MITF and phosphotylated ERK1 and ERK2 were used for immunodetection.
As shown in Figure 2, the addition of oligopeptide-68 (0.1 µg/mL) to cell cultures in the absence of any other stimulus rapidly reduced the protein levels of MITF. Since they depend upon MITF, tyrosinase protein levels are also reduced by oligopeptide-68. On the other hand, phosphorylation levels of ERK1 and ERK2 appear to inversely correlate with MITF expression. These results were obtained within a short period of time (30 sec), suggesting the proteolytic degradation of these proteins in the presence of oligopeptide-68.
The fact that the expressions of MITF, tyrosinase, TRP-1 and TRP-2 proteins are down-regulated by oligo-peptide-68 alone, in the absence of external stimuli, suggests the material is able to modulate the constitutive skin pigmentation pathway.
RT-PCR analysis: B16-F10 cells were cultured for 24 hr before treatment with serum-free media at 37°C under 5% carbon dioxide. To mimic what happens during sun exposure, researchers then stimulated melanocytes with α-MSH for 2 hr in the presence or absence of increasing concentrations of oligopeptide-68. The total RNA was isolated from cells and transcribed to DNA using reverse transcriptase. The resulting cDNAs served as templates for subsequent PCR amplification using primers specific for MITF, TRP-1 and TRP-2 genes. Detection of β-actin DNA was used as an internal standard.
The results show that the expressions of MITF, TRP-1 and TRP-2 were strongly induced with α-MSH treatment (see Figure 3). Interestingly, the addition of oligopeptide-68 abolished the capacity of α-MSH to stimulate MITF, TRP-1 and TRP-2 gene expression. This effect was dose-dependent. From this experiment, the authors concluded that oligopeptide-68 is able to inhibit the facultative pigmentation pathway by opposing MITF, TRP-1 and TRP-2 gene expression induced by α-MSH.
Evaluation of melanin content: B16-F10 cells were cultured for 48 hr before treatment with 2% serum media at 37°C under 5% carbon dioxide conditions. Cells were then stimulated with α-MSH (100 ng/mL), in the presence or absence of increasing concentrations of oligopeptide-68 for four days. Cells were trypsinized, washed in PBS, pelleted by centrifugation, and solubilized in 1M sodium hydroxide. Melanin content was measured spectrophotometrically at 405 nm.
As expected, α-MSH treatment of the melanocytes resulted in increased melanin deposition, as shown in Figure 4. Further, the addition of oligopeptide-68 to the incubation media dose-dependently inhibited this α-MSH-induced increase in pigment formation. At the strongest concentration used (10 mg/mL), oligopeptide-68 reduced the melanin content to slightly below baseline levels, again suggesting its effects on constitutive pigmentation as well as on α-MSH driven pigmentation.
Clinical Studies
An in vivo study was conducted with a panel of 23 healthy Asian women, ages 33–55, presented with at least one hyperpigmented spot. Volunteers applied a test serum containing the encapsulated oligopeptide-68 (see Formula 1) twice daily on the whole face for 56 days. Colorimetric measurements were taken of the designated pigmented spots using a spectrophotometer. Analysis of the colorimetric parameter L*, indicating the perceived brightness compared with perfect white, was conducted before the experiment and on days 28 and 56 of the experiment. Under this protocol, a lightening effect translates as an augmentation in the L* parameter. Application of encapsulated oligopeptide-68 resulted in a steady and statistically significant augmentation of the average L* parameter in time.
The increase from baseline (control) was evaluated at 1.9% at day 28 and continued to increase, reaching 2.9% at day 56. The constant improvement attests to a building effect of the lightening benefits of oligopeptide-68 with time. Most interestingly, all participants showed a noticeable degree of improvement in skin lightness.
In the same study, volunteers were analyzed by trained dermatologists to assess the changes in skin color of the designated spots before the study and at days 28 and 56 of the study. A color scale ranging from 1, for very pale skin, to 10 for very dark skin was used. Results, shown in Figure 5, documented an average reduction in skin darkness of 21% at day 28, increasing to 30% at day 56. Again, a building effect of the lightening of encapsulated oligopeptide-68 was documented with time.
Figure 6 shows a before-and-after photograph of a volunteer from the above study. The green arrow points to a hyperpigmented area that was monitored throughout the study. At day 56 of treatment with encapsulated oligopeptide-68, a noticeable reduction in the contour and color of the selected spots was observed for this volunteer.
Conclusion
Skin pigmentation is a complex symphony in which a number of performers are mobilized. When the music turns cacophonous or the absence of music is desired, various aspects of the performance must be simultaneously addressed. While several lightening/whitening ingredients target different sections of the skin pigmentation orchestra, proper combinations should complement one another to modulate the whole process in harmony. Formulating with encapsulated oligopeptide-68 could be part of such protocols since it acts on key players of the melanogenic music including MITF, the orchestra leader.
Encapsulated oligopeptide-68 can simultaneously address both constitutive and facultative pigmentation. Specifically, in vitro studies link the effects of oligopeptide-68 on constitutive pigmentation to a rapid decline in the levels of the expression of MITF, tyrosinase, TRP-1 and TRP-2 proteins. These effects are mediated through activation of the RAS/ERK signaling pathway. Further, modulation of facultative pigmentation by the peptide involves a reduction in α-MSH-induced gene transcription of MITF, TRP-1 and TRP-2. In vitro, oligopeptide-68 has thus proven to abrogate the stimulatory effects of α-MSH on melanin pigment formation.
Clinical results demonstrated that encapsulated oligopeptide-68 has significant and progressive whitening/lightening effects. In addition, a complete evaluation of the ocular and cutaneous tolerance of oligopeptide-68 showed the peptide to exhibit an excellent safety profile (data not shown). Nevertheless, to optimize the benefits of such a product, sun avoidance and/or SPF protection is mandatory.
References
Send e-mail to: [email protected].
- JH Chung, Photoaging in Asians, Photo- dermatol Photoimmunol Photomed 19(3)109–121 (2003)
- Brightening skin care stretches whitening to new markets, Mintel report (Jul 2009)
- M Mullick, Country focus: India, Indian Society of Cosmetic Chemists presentation at In-Cosmetics Asia (2010)
- Skin lighteners: A global strategic business report (MCP-6140), announced by Global Industry Analysts Inc. (Jul 2009)
- MG Kosmadaki, A Naif and P Hee-Young, Recent progresses in understanding pigmentation, G Ital Dermatol Venereol 145(1) 47–55 (2010)
- Y Yamaguchi and VJ Hearing, Physiological factors that regulate skin pigmentation, Biofactors 35(2) 193–199 (2009)
- Y Cheli, M Ohanna, R Ballotti and C Bertolotto, Fifteen-year quest for microphthalmia-associated transcription factor target genes, Pigment Cell Melanoma Res 23(1) 27–40 (2010)
- R Buscà and R Ballotti, Cyclic AMP a key messenger in the regulation of skin pigmentation, Pigment Cell Res 13(2) 60–69 (2000)
- M Martínez-Esparza, C Jiménez-Cervantes, F Beermann, P Aparicio, JA Lozano and JC García-Borrón, Transforming growth factor-beta1 inhibits basal melanogenesis in B16/F10 mouse melanoma cells by increasing the rate of degradation of tyrosinase and tyrosinase-related protein-1, J Biol Chem 272(7) 3967–3972 (1997)
- DS Kim, SH Park and KC Park, Transforming growth factor-beta1 decreases melanin synthesis via delayed extracellular signal-regulated kinase activation, Int J Biochem Cell Biol 36(8) 1482–1491 (2004)
- PT Pugliese, Pigmentation revisited, Skin Inc. 21(3) 68–74 (2009)
- SW Hwang, DJ Oh, D Lee, JW Kim and SW Park, Clinical efficacy of 25% L-ascorbic acid (C’ensil) in the treatment of melasma, J Cutan Med Surg 13(2) 74–81 (2009)
- K Sato, M Morita, C Ichikawa, H Takahashi and M Toriyama, Depigmenting mechanisms of all-trans retinoic acid and retinol on B16 melanoma cells, Biosci Biotechnol Biochem 72(10) 2589–2597 (2008)
- D Simonot, J McColl and D Thome, Tyrosinase inhibitors: Activity of a rumex extract in combination with kojic acid and arbutin, Cosm & Toil 117(3) 52–56 (2002)
- HJ Kim et al, Visualization of the melanosome transfer inhibition in a mouse epidermal cell co-culture model, Int J Mol Med 25(2) 249–253 (2010)