Increased melanin pigmentation is a physiological mechanism that the skin adopts to protect itself from the damaging effect of sustained and prolonged UV light exposure. Melanin pigment, produced by melanocyte cells in the basal layers of the epidermis, is transferred to the keratinocytes in the epidermis and sits on the top of the keratinocyte’s nucleus to protect the cell’s DNA. However, in some conditions (i.e., inflammation or a hormonal imbalance) and with increasing age, the deposition of melanin in the epidermis increases. This is particularly evident in extreme cases such as melasma, where patchy melanin formation on skin is observed.1 Skin inflammation and sustained damage can also be a fundamental trigger for excessive pigmentation,2, 3 with clinical relevance in the case of post inflammatory pigmentation.4 Other examples of increased pigmentation due to skin irritation and/or inflammation are documented in women developing axillary melanin spots due to hair removal irritation5 or in individuals suffering from acne.6
Those experiencing skin pigmentation as a result of inflammation or irritation are often associated with phototypes III–IV on the Fitzpatrick Scale. In order to even skin tone, decrease skin pigmentation and reduce the formation of dark spots, a series of lightening and depigmenting agents have been developed over the years targeting different steps of the melanogenesis process.7, 8 Among the agents most utilized for this purpose are: hydroquinone, kojic acid, arbutin, vitamin C, etc. However, many of these agents have been questioned for their safety7, 9 and have been restricted for their use in many countries. Therefore novel skin lighteners and depigmenting agents with a proven efficacy and safety profile remain a prominent need in personal care.
Materials
The root Rheum rhaponticum, a species of rhubarb, was recently investigated for its high content of polyphenols, specifically for its hydroxystilbenes.10 Stilbenes are small molecules occurring naturally in several plants, with resveratrol the most well-known and most studied stilbene. Resveratrol and hydroxystilbenes have been extensively investigated and associated to many beneficial properties (antioxidant, antiinflammatory and anti-aging) in vitro,11, 12 in animals13, 14 and in humans.15, 16 Hydroxystilbenes and their derivatives have been investigated also for their effect on melanogenesis through their activity on the enzyme tyrosinase, which allows them to inhibit melanin formation, 17, 18 but also possibly through their antioxidant activity.19 The inhibitory activity on melanin formation and antiinflammatory properties was observed when extracts from different Rheum species were tested.20-22
Based on this research, a highly concentrated extract of hydroxystilbenes (60–70%) was isolated from the root of R. rhaponticum. Through a proprietary technology, the hydroxystilbenes extract is further acetylated. There is some evidence in literature that acetylation of polyphenols may increase their biological activity,23 with stilbene resveratrol being a notable example.24, 25 The resulting acetylated hydroxystilbenes extract was dissolved in panthenyl triacetate (PTA) to create a novel complex (R. rhaponticum complex)a. PTA was used due to its solubilization properties and to its added value as a natural anti-irritant and anti-inflammatory molecule in its active form.26–28 The association between melanin formation and inflammation therefore allows an anti-irritant molecule such as PTA an optimal complement to the acetylated stilbenes extract.
Experimental Methods
These studies first tested whether acetylation of the hydroxystilbenes extract from R. rhaponticum increased the extract’s effect on melanogenesis. The R. rhaponticum complex was then tested for its capacity to interfere with melanogenesis in human skin explants and in a clinical study (double-blind, against a placebo), for its activity on pigmented spots and on skin tone. Finally, the activity of the complex on pigmented spots and on skin tone was measured to see if it could correlate with an effect on skin radiance, as chromophores such as melanin orientate and absorb light, which influences the optical properties of the skin and results in skin radiance.29, 30
In vitro studies on B16 melanocytes: B16 murine melanocytes were seeded in 96-well plates and cultured for 24 hr. The culture medium was then replaced with a culture medium supplemented with the stable μ-MSH analog Nle4,DPhe7-μ-MSH (NDPMSH) to stimulate melanocytes and either kojic acid (50 μg/ml), R. rhaponticum extract (50 μg/ml) or acetylated R. rhaponticum extract (50 μg/ml). A non-stimulated control condition in absence of NDP-MSH was performed in addition to solvent controls (DMSO in presence of NDP-MSH). The melanocytes were incubated for 72 hr. All experimental conditions were performed in triplicates. At the end of the incubation, the total quantity of melanin (extracellular and intracellular) was evaluated by measuring the absorbance at 405 nm of each sample and normalizing the values using a range of melanin standards.
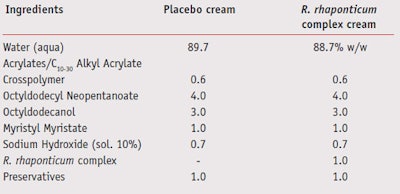
Ex vivo studies on human skin explants: Six human skin explants (10 mm diameter/each) were prepared from a plastic surgery biopsy. The explants were kept in culture medium at 37°C, 5% CO2. Explants were then divided for experimental condition treatments and UV-irradiated (UVA 1.125 J/ cm² with 6–8% UVB, which were measured with a radiometerb) for six days. Treatments were every other day for six days with a 2 mg/cm2 cream formulated with or without 1% R. rhaponticum complex, as shown in Table 1. As a benchmark, kojic acid was used (0.15% water solution). Untreated, irradiated and non-irradiated controls were run and six explants per condition were used. At day six, explants were fixed in formalin, dehydrated and embedded in paraffin. Several 5 μm cuts were realized (at least 2–3/explant). Subsequently, melanin staining was found by argent embedding using the Masson/Fontana technique.
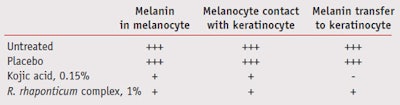
A semi-quantitative evaluation of melanin in melanocytes, melanocyte contact with keratinocytes and melanin transfer in keratinocytes was then performed through microscopical analysisc, and images were registeredd. A score was created to quantify the observations. To quantitatively evaluate melanin accumulation in the keratinocytes basal layer (melanin %), image analysis was performede.
In vivo clinical evaluation: An experimental panel comprised of 20 healthy Caucasian female subjects 35–65 years with phototype Fitzpatrick II–III was selected under the supervision of a dermatologist. These subjects exhibited visible brown pigmented spots on the face due to excessive sun exposure and/or due to chronological aging. In addition, they were not pregnant or breast-feeding; they did not have allergies or sensitivity to cosmetic products, toiletries, sunscreens and/or topical drugs; and they did not have positive anamnesis for atopy. Instructions on how to use the products, the informative form of the study and the products to be tested were then given. The study was double-blind.
A cream-gel placebo and a cream-gel containing 1% of the R. rhaponticum complex (Table 1) were applied on either half of the subject’s face. Products were applied twice per day (morning and evening), and their effects were evaluated at 15, 30 and 60 days. Comparison was made at the start of the study (time zero [T0]) between the placebo cream and the cream formulated with the R. rhaponticum complex.
Measurement of the melanin index (see Melanin Index) in the pigmented spots on the face was conducted by evaluating skin color with a measurement probef. Measurement of skin tone and skin radiance was performed by using a spectrophotometer/colorimeterg. For skin tone, the value L* that defines the color brightness according to the CIELab standardized color space was measured. For skin radiance, the amount of light reflected at an 8-degree specular angle from the skin surface was registered. The information was then elaborated by a microprocessor and quantified.
Results
In vitro and ex vivo data: Incubation of 50 μg/ml R. rhaponticum hydroxystilbenes extract with NDP-MSH induced B16 melanocytes produced a clear inhibition of melanin synthesis (both intracellular and secreted) as shown in Figure 1. The inhibition was statistically significant compared to the stimulated but untreated control (p<0.001, Student’s t-test). Most interestingly, the acetylated R. rhaponticum hydroxystilbenes extract was the most effective in inhibiting melanin production (-86% vs stimulated control), superior to the non acetylated R. rhaponticum hydroxystilbenes and to kojic acid (50 μg/ml), used as a melanin synthesis inhibitor reference.
When the R. rhaponticum complex was incorporated in a cream at 1% and applied on UV-treated full thickness human skin explants, it was able to reduce pigmentation steps as assessed by semi-quantitative (microscopic observation followed by score) and quantitative (microscopic observation followed by image analysis) evaluation. UV-irradiation for six consecutive days of human skin explants produced an increase in melanin synthesis in all explants when compared to nonirradiated ones (data not shown). In Figure 2, histology cuts with the different treatments are shown. From these images, melanin localization can be seenin the keratinocytes’ basal layer, on the top of the nuclei. Based on these images, treatment differences were evaluated with microscopic observations followed by a score (see experimental methods). Results are summarized in Table 2. While the placebo cream treatment did not affect (at this level of observation) all the parameters observed, both the kojic acid cream and the cream containing the R. rhaponticum complex affected melanin synthesis, the melanocyte’s contact with basal layer keratinocytes and melanin transfer to keratinocytes.
When image analysis for % melanin in keratinocytes’ basal layer was concluded, the different treatments were proven to inhibit melanin accumulation, as shown in Figure 3. All treatments were effective compared to untreated irradiated control (p<0.001, Student’s t-test), with the cream containing the R. rhaponticum complex being the most effective, as it reduced melanin content by 48% compared to the placebo. (p<0.001, Student’s t-test).
In vivo clinical study data: A panel was run to test the activity of a cream with or without the R. rhaponticum complex by daily measuring melanin index in pigmented spots (Figure 4), skin brightness (Figure 5) and skin radiance (Figure 6) daily. In Figure 4, the cream containing the R. rhaponticum complex was six times more effective at reducing melanin index than the placebo cream after 15 days (p<0.05 compared to placebo, Student’s t-test). In Figure 5, the cream containing the R. rhaponticum complex was almost three times more effective than the placebo cream and significantly different after 30 and 60 days of treatment (p<0.05 compared to placebo, Student’s t-test), with a resulting skin brightness increase. In Figure 6, the cream containing the R. rhaponticum complex was more than double the placebo cream in increasing skin radiance after 30 days (p<0.05 compared to placebo, Student’s t-test). Overall, the clinical study confirmed the capacity of the R. rhaponticum complex to decrease melanogenesis by reducing pigmentation and by increasing skin brightness with the final result of enhancing skin radiance.
Discussion and Conclusions
This paper illustrates that the R. rhaponticum complex is a novel potent melanogenesis inhibitor. This combination has shown activity against melanogenesis, in UV-induced human skin explants when introduced at 1% in a cream. The activity of the R. rhaponticum complex was shown to be more effective than both a placebo treatment and a kojic acid reference. Finally, the melanogenesis inhibition was correlated, in double-blind clinical studies using the R. rhaponticum complex at 1% in a cream, to an increase in skin brightness, to reduced pigmentation and to enhanced skin radiance. Differences were statistically significant when compared to the placebo cream.
Interestingly, in vitro testing in hormone-induced B16 melanocytes (Figure 1) showed that the acetylation step on the hydroxystilbenes extract was associated with an increased melanogenesis inhibition. Although the possible reason and mechanism behind this effect was not explored experimentally, it is possible that the chemical modification on the hydroxyl groups by adding acetyl groups may have increased the bioavailability or the intracellular stability of the compounds resulting in an augmented potency, as previously observed with cathechins.23 It is also possible that the acetylation may have increased their biological activity through a different mechanism, as observed in previous studies when resveratrol was acetylated.24, 25
When 1% of the R. rhaponticum complex in a cream and kojic acid at 0.15% in water were tested in UV-induced human skin explants and their effect was measured using a semi-quantitative score, they both proved effective at reducing melanogenesis (Table 2). However, when a quantitative evaluation was done by measuring melanin accumulation in basal layer keratinocytes, the R. rhaponticum complex was clearly superior to kojic acid (-48% and -18%, respectively compared to placebo). It is possible that the concentration of kojic acid used during this study was not high enough to induce a higher inhibition on melanin deposition, as suggested by a recent study.9 However, both semiquantitative and quantitative evaluation of the R. rhaponticum complex activity on melanogenesis confirm its inhibitory effect (Figure 3).
The clinical study illustrated that the R. rhaponticum complex at 1% in a cream can have a skin brightening effect (increase of L* value) when applied on the face of human volunteers. This effect was already observed after 15 days of application and became statistically significant compared to a placebo treatment after 30 and 60 days of application (Figure 5). The results correlate well with the anti-melanogenesis activity of the R. rhaponticum complex and of the acetylated hydroxystilbenes extract as one of the components, as observed in ex vivo and in vitro studies. In this regard, the R. rhaponticum complex contributes to a brighter, fairer skin tone.
Further observation on human volunteers showed that the R. rhaponticum complex can also act as a de-pigmenting agent when applied on pigmented spots on the face of human volunteers. This effect was already observed after 15 days and statistically significant compared to a placebo treatment, remaining significant over 30 and 60 days of application (Figure 4). Based on this data, it is possible that the R. rhaponticum complex not only reduces novel melanin formation but also influences melanin removal from pigmented spots or melanin transfer from the basal layer to the upper layers. This hypothesis would need to be investigated further.
Finally, the R. rhaponticum complex was able to increase skin radiance on human volunteers and the effect was significant compared to a placebo treatment after 30 and 60 days of application (Figure 6). The correlation between melanin formation and skin optical properties have been studied,29, 30 and it is not surprising that a reduced melanin index and an increased L* value (indicator of skin brightness) would translate to a different skin optical behavior with respect to light reflection, transmission and diffusion that, in this case, translate in an increased skin radiance.
In conclusion, the R. rhaponticum complex has proven to be an effective skin-brightening agent and inducer of skin radiance in human volunteers. Its utilization in topical treatment for skin brightening, anti-pigmentation, radiance boosting is suggested in individuals who need to address skin discoloration; in homogeneous skin tone and complexion; dull, lackluster skin; or in individuals suffering of mild melasma or excessive pigmentation.
The R. rhaponticum complex has been tested for skin tolerance, mutagenicity and biodegradability, and has exhibited an excellent safety profile. It is easy to formulate with and is recommended at 0.5–1.0% in brightening products, to rebalance skin discoloration and to reduce excessive skin pigmentation.
References
1. YC Kauh and TF Zachian, Melasma, Adv Exp Med Biol 455 491–499 (1999)
2. M Prunieras, Melanocytes, melanogenesis, and inflammation, Int J Dermatol 25 624–628 (1986)
3. PC Eves, S MacNeil and JW Haycock, alpha- Melanocyte stimulating hormone, inflammation and human melanoma, Peptides 27 444–452 (2006)
4. S Taylor, P Grimes, J Lim, S Im and H Lui, Postinflammatory hyperpigmentation, J Cutan Med Surg 13 183–191 (2009)
5. AG James, JE Pople, WE Parish, AE Moore and N Dunbar, Histological evaluation of hyperpigmentation on female Filipino axillary skin, Int J Cosmet Sci 28 247–253 (2006)
6. SK Shah and AF Alexis, Acne in skin of color: practical approaches to treatment, J Dermatolog Treat 21 206–211 (2010)
7. ZD Draelos, Skin lightening preparations and the hydroquinone controversy, Dermatol Ther 20 308–313 (2007)
8. JM Gillbro and MJ Olsson, The melanogenesis and mechanisms of skin-lightening agentsexisting and new approaches, Int J Cosmet Sci 33 210–221 (2011)
9. CL Burnett, WF Bergfeld, DV Belsito, RA Hill, CD Klaassen, DC Liebler, JG Marks Jr, RC Shank, TJ Slaga, PW Snyder and FA Andersen, Final report of the safety assessment of Kojic acid as used in cosmetics, Int J Toxicol 29 244–273 (2010)
10. T Püssa, P Raudsepp, K Kuzina and A Raal, Polyphenolic composition of roots and petioles of Rheum rhaponticum L, Phytochem Anal 20 98–103 (2009)
11. J Jagdeo, L Adams, H Lev-Tov, J Sieminska, J Michl and N Brody, Dose-dependent antioxidant function of resveratrol demonstrated via modulation of reactive oxygen species in normal human skin fibroblasts in vitro, J Drugs Dermatol 9 1523–1526 (2010)
12. Y Matsui, K Sugiyama, M Kamei, T Takahashi, T Suzuki, Y Katagata and T Ito, Extract of passion fruit (Passiflora edulis) seed containing high amounts of piceatannol inhibits melanogenesis and promotes collagen synthesis, J Agric Food Chem 58 11112– 11118 (2010)
13. A Raal, P Pokk, A Arend, M Aunapuu, J Jõgi, K Okva and T Püssa, Trans-resveratrol alone and hydroxystilbenes of rhubarb (Rheum rhaponticum L.) root reduce liver damage induced by chronic ethanol administration: a comparative study in mice, Phytother Res 23 525–532 (2009)
14. JK Kundu, YK Shin, SH Kim and YJ Surh, Resveratrol inhibits phorbol ester-induced expression of COX-2 and activation of NFkappaB in mouse skin by blocking IkappaB kinase activity, Carcinogenesis 27 1465–1474 (2006)
15. KA Roupe, CM Remsberg, JA Yáñez and NM Davies, Pharmacometrics of stilbenes: seguing towards the clinic, Curr Clin Pharmacol 1 81–101 (2006)
16. N Elmali, O Baysal, A Harma, I Esenkaya and B Mizrak, Effects of resveratrol in inflammatory arthritis, Inflammation 30 1–6 (2007)
17. K Ohguchi, T Tanaka, T Kido, K Baba, M Iinuma, K Matsumoto, Y Akao and Y Nozawa, Effects of hydroxystilbene derivatives on tyrosinase activity, Biochem Biophys Res Commun 307 861–863 (2003)
18. YM Kim, J Yun, CK Lee, H Lee, KR Min and Y Kim, Oxyresveratrol and hydroxystilbene compounds. Inhibitory effect on tyrosinase and mechanism of action, J Biol Chem 277 16340–16344 (2002)
19. T Yokozawa and YJ Kim, Piceatannol inhibits melanogenesis by its antioxidative actions, Biol Pharm Bull 30 2007–2011 (2007)
20. KT Lee, BJ Kim, JH Kim, MY Heo and HP Kim, Biological screening of 100 plant extracts for cosmetic use (I): inhibitory activities of tyrosinase and DOPA auto-oxidation, Int J Cosmet Sci 19 291–298 (1997)
21. K Iida, K Hase, K Shimomura, S Sudo, S Kadota and T Namba, Potent inhibitors of tyrosinase activity and melanin biosynthesis from Rheum officinale, Planta Med 61 425–428 (1995)
22. TM Ngoc, PT Minh, TM Hung, PT Thuong, I Lee, BS Min and K Bae, Lipoxygenase inhibitory constituents from rhubarb, Arch Pharm Res 31 598–605 (2008)
23. JD Lambert, S Sang, J Hong, SJ Kwon, MJ Lee, CT Ho and CS Yang, Peracetylation as a means of enhancing in vitro bioactivity and bioavailability of epigallocatechin-3-gallate, Drug Metab Dispos 34 2111–2116 (2006)
24. E Fragopoulou, T Nomikos, HC Karantonis, C Apostolakis, E Pliakis, M Samiotaki, G Panayotou and S Antonopoulou, Biological activity of acetylated phenolic compounds, J Agric Food Chem 55 80–89 (2007)
25. D Colin, A Lancon, D Delmas, G Lizard, J Abrossinow, E Kahn, B Jannin and N Latruffe, Antiproliferative activities of resveratrol and related compounds in human hepatocyte derived HepG2 cells are associated with biochemical cell disturbance revealed by fluorescence analyses, Biochimie 90 1674–1684 (2008)
26. K Biro, D Thaçi, FR Ochsendorf, R Kaufmann and WH Boehncke, Efficacy of dexpanthenol in skin protection against irritation: a doubleblind, placebo-controlled study, Contact Dermatitis 49 80–84 (2003)
27. E Prokscha and HP Nissen, Dexpanthenol enhances skin barrier repair and reduces inflammation after sodium lauryl sulphateinduced irritation, J Dermatolog Treat 13 173–178 (2002)
28. F Ebner, A Heller, F Rippke and I Tausch, Topical use of dexpanthenol in skin disorders, Am J Clin Dermatol 3 427–433 (2002)
29. SH Tseng, P Bargo, A Durkin and N Kollias, Chromophore concentrations, absorption and scattering properties of human skin in-vivo, Opt Express 17 14599–14617 (2009)
30. GE Piérard and E Uhoda, Skin photophysics and colors, Rev Med Liege 60 48–52 (2005)