The natural personal care market has seen strong growth since 2008, which is projected to continue worldwide.1 To meet consumer demand for natural personal care products, leading brands will continue to develop botanically derived products that can be produced sustainably and organically. Moringa oleifera Lam. has been used for centuries for general well-being, nutrition, healing and skin care.2 Its multi-faceted uses have led to regional monikers including “miracle tree” and “tree for purifying” in the Nile Valley, and “mother’s best friend” in the Philippines.3, 4 Historically, moringa seed oil was used by the Romans, Greeks and Egyptians as skin lotions and perfumes. Today, it is used for skin and hair care.5, 6 However, while the cosmetic industry has employed oil from its seed, which has demonstrated relatively high oxidative stability,7, 8 extracts from the leaves remain an untapped source for enhancing skin health.
Moringa plants are fast-growing, drought-resistant, deciduous trees averaging ~12 meters in height.9 The leaves of the plant provide a rich source of protein, vitamin C, β-carotene, calcium and potassium, and are advocated as a nutrient rich food to combat malnutrition in many areas of the world.2 The trees are native to the western and sub-Himalayan tracts of India, and have spread throughout tropical and subtropical regions of Africa, Asia and the Americas.3, 8 Moringa cultivation has further expanded into India, the Philippines and the United States (Hawaii).
The density of nutrients and chemical stability of phytoactives makes moringa well-suited as a naturally sourced cosmetic ingredient. Additionally, experimental and anecdotal evidence exists for skin-related applications of moringa such as treating topical wounds and conditions that require antibacterial activity. In the Central American countries of Nicaragua and Guatemala, moringa leaves are applied as a poultice on sores, and seed oil is applied externally for skin diseases.10 In addition, an aqueous extract of moringa leaves has been reported to promote wound healing in vivo,11 and fresh leaf juice and aqueous extract from the seeds were found to inhibit the growth of Pseudomonas aeruginosa and Staphylococcus aureus in vitro.12
In addition to high levels of nutrients, moringa leaves can form large quantities of phytoactive compounds including four uniquely stable isothiocyanates (see Figure 1). These are produced by crushing and incubating leaves in aqueous solution to release the plant enzyme myrosinase from cellular compartments, catalyzing the conversion of naturally occurring glucosinolates to isothiocyanates (see Figure 2). Moringa isothiocyanates (MICs) are structural and functional analogues of sulforaphane, the well-studied but unstable isothiocyanate from broccoli known to convey strong cytoprotective activity.13
Sulforaphane is one of the most potent isothiocyanates; it exerts cancer chemopreventive and cytoprotective effects mainly by inducing genes that encode phase 2 detoxifying and antioxidant enzymes.14, 15 Thus, sulforaphane acts as an indirect antioxidant that upregulates cytoprotective proteins and glutathione levels via nuclear factor erythroid 2-related factor 2 (Nrf2) activation.16 Sulforaphane has also been shown to protect mouse and human keratinocytes against oxidative stress caused by chemical oxidants and UVA radiation.17, 18 It was therefore hypothesized that isothiocyanates from moringa could confer skin-protecting benefits through the induction of cytoprotective enzymes.
Moringa’s Phytoactive Compounds
Previously published research has shown moringa concentrate (MC) to contain 1.7% isothiocyanates as well as 3.8% polyphenols. Regarding stability, MC was stored at 25°C or 37°C for up to 30 days, and MICs were quantified by high performance liquid chromatography. The major isothiocyanates MIC-1 and MIC-4 (see Figure 1) maintained 70% and 100% stability, respectively, after storage at room temperature. At 37°C, MIC-4 demonstrated 80% stability, while MIC-1 only demonstrated 20% stability.19 In comparison with sulforaphane, which rapidly degrades,13 moringa isothiocyanates may therefore exhibit a stability advantage.
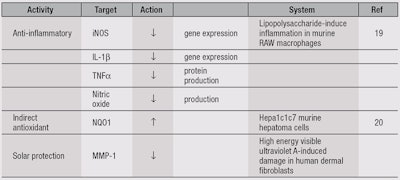
MC, and specifically MIC-1 and MIC-4, also demonstrated anti-inflammatory activity, inhibiting lipopolysaccharide (LPS)-induced inflammation in a macrophage cell line in a dose-dependent manner (see Table 1).19 The gene expression of inducible nitric oxide synthase (iNOS) and interleukin-1β (IL-1β), as well as the production of nitric oxide and tumor necrosis factor α (TNFα) protein, were also significantly reduced by MC (5-100 µg/mL) and MICs (1 and 5 µM), in comparison with LPS-induced vehicle (50% ethanol) control.19
Furthermore, MC and isothiocyanates isolated from moringa have shown direct and indirect antioxidant activity.20 Antioxidant protection is distinguished functionally as either direct or indirect. Direct activity refers to small molecular antioxidants that are redox-active and consumed in the reaction, whereas indirect antioxidants are not consumed in the reaction—and therefore may have longer-lasting effects.21
Direct antioxidant activity of MC was measured using the oxygen radical absorbance capacity (ORAC) assay, which assesses quenching of the peroxyl radical generated by 2,2’-azobis(2-amidinopropane)dihydrochloride (AAPH), and is reported as equivalents to the antioxidant Trolox. The direct antioxidant activity of MC, likely derived from its polyphenolic components, was 3.6 mmol Trolox equivalents/g19—greater than that of many spices commonly ranked as top antioxidants, such as cinnamon, clove and oregano.22
Further research revealed that MC, and in particular MIC-1 and MIC-4, demonstrated indirect antioxidant activity.20 Indirect antioxidants induce cytoprotective proteins (phase 2 enzymes) such as NAD(P)H quinone oxidoreductase 1 (NQO1), glutathione transferase (GST) and UDP-glucuronosyltransferase (UGT).21 Both MICs were as potent as sulforaphane, at a physiologically-relevant concentration of 5 µM, in inducing activity of NQO1 in Hepa 1c1c7 mouse hepatoma cells (see Table 1).20 Thus, MC was shown to have bifunctional antioxidant properties, providing direct antioxidant activity from polyphenol components and indirect antioxidant activity from the isothiocyanates.
The present paper next investigates the capability of a moringa concentrate to confer UV protection benefits. Increased levels of matrix metalloproteinase-1 (MMP-1), a collagen degrading enzyme, are believed to be associated with wrinkle formation during the aging process, and its expression is accelerated by solar damage.23 Thus, the expression of MMP-1 was measured in cultured human dermal fibroblasts, as described here.
Materials and Methods
Extract preparation: A proprietary extraction technique was used to develop a water-soluble moringa concentratea containing high MIC levels not otherwise present in moringa-derived ingredients.19 Briefly, fresh moringa leaves were homogenized in an aqueous suspension 1:5 (w/v) and incubated at room temperature (22°C) for 30 min. This suspension was filteredb and centrifuged for 10 min at 3200 × g and 4°C to produce a clarified solution. MIC-1 and MIC-4 were isolated by high performance liquid chromatography and structures were confirmed by liquid chromatography mass spectrometry and 1H NMR spectroscospy.19
Key to the development of the MC is the proprietary aqueous extraction process, which as described, allows the release of the plant enzyme myrosinase from the moringa leaves to catalyze the conversion of glucosinolates into stable isothiocyanates (see Figure 1). Specifically, myrosinase hydrolyzes the thioglucose linkage of a glucosinolate, forming an aglycone intermediate, which undergoes chemical rearrangement to form an isothiocyanate.24 Extracts made from fresh moringa leaves preserve and utilize myrosinase activity, whereas in commercially available moringa leaf powders, myrosinase is inactivated in the drying process.24
HDFn protocol: Neonatal human dermal fibroblasts (HDFn)c were treated with high energy visible and ultraviolet A light (HEV/UVA) to induce MMP-1 expression, followed by treatment with MC or MICs to evaluate UV protective effects. HDFn were maintained at 37°C with 5% CO2 in Dulbecco’s Modified Eagle Medium (DMEM)d supplemented with 10% (v/v) fetal bovine serum, 100 I.U. mL-1 penicillin and 100 mg L-1 streptomycine.
For experiments, HDFn were plated in 60-mm Petri dishes and grown to 95% confluence. Cells were washed with phosphate buffer solution (PBS) and incubated in serum-free DMEM for 24 hr. Prior to treatment, cells were washed again with 1× PBS, 5 mL of PBS was added, and uncovered cells were placed 28 cm from the light source and treated with HEV/UVAf for 11 min, for a dose of 11 J/cm2.
After HEV/UVA treatment, the PBS was removed and cells were incubated in 4 mL DMEM with one of the following treatments for 24 hr: 50% ethanol vehicle, 1 µM retinoic acid, 10 µg/mL MC, 1 µM MIC-1 or 1 µM MIC-4, then media and RNA were collected.
An additional plate without HEV/UVA treatment was included in each experimental replicate to serve as a baseline control. For cell viability experiments, cells were treated under the same conditions and 100 µg/mL MTTg was added to the media after the 24-hr sample treatment step, then incubated for an additional 3-4 hr. Media was then removed, and formazan crystals were dissolved in dimethylsulfoxide. The absorbance of formazan was recorded at 570 nm using a microplate readerh to determine cell viability. Passage 4-9 cells were used for all experiments.
RNA extraction, cDNA synthesis and RT-PCR: Total RNA was extracted from HDFn using a reagentj following the manufacturer’s instructions. RNA clean-up columnsk were used to improve RNA quality, the quantification of which was analyzed using a sample retention systemm. A ratio of OD 260/280 ≥ 2.0 and 260/230 ≥ 1.8 was considered to be good quality RNA. RNA was DNAse treatedn, and 1 μg of RNA was used for first strand cDNA synthesisp.
The thermal cycle program was set as follows: 10 min at 25°C; 60 min at 37°C; 60 min at 37°C; 5 sec at 85°C; and final hold at 4°C. cDNAs were diluted tenfold and 5 μL of the dilution was used for polymerase chain reaction (PCR) with 12.5 μL of PCR amplification reagentq, 0.5 μL of primer dilution (6 μM) and 7 μL biotechnology performance certified (BPC)-grade waterr to a final reaction volume of 25 μL.
Primer sequences were as follows: MMP-1 (RefSeq: NM_002421): forward primer 5’-TGG GCT GTT CAG GGA CAG A-3’, reverse primer 5’-TTC ACA GTT CTA GGG AAG CCA AA-3’; SDHA, succinate dehydrogenase complex, subunit A, (RefSeq: NM_004168): forward primer 5’-GGA AGC ATA AGA ACA TCG GAA CTG-3’, reverse primer 5’-CTG ATT TTC CCA CAA CCT TCT TGC-3’.25, 26 Primers were validated before use. Quantitative real time-PCR amplifications were performeds with the following thermal cycler profile: 10 min at 95°C; 15 sec at 95°C for 40 cycles; and 1 min at 60°C. No template control and non-reverse transcription control were included in each qRT-PCR reaction for quality control.
MMP-1 mRNA expression levels were analyzed using the comparative ΔΔCt method and normalized with respect to the average Ct value of SDHA. MMP-1 expression levels were normalized to HEV/UVA-induced control. Relative gene expression values ≤1 indicated gene inhibition and values ≥ 1 indicated over-expression, in excess of HEV/UVA induction. Data was evaluated by one-way analysis of variance followed by Fisher’s least significance difference testt. A p value < 0.05 was considered statistically significant compared to HEV/IVA treated control.
Results and Discussion
Compared with HEV/UVA-induced control cells, MC significantly inhibited the HEV/UVA-induced gene expression of MMP-1 in cultured human dermal fibroblasts (see Figure 3) by 43%, at a dose demonstrating no cytotoxicity (10 µg/mL). Retinoic acid 1 µM, used as a positive control, inhibited MMP-1 expression by 33%, compared with control cells. Interestingly, however, treatment with MIC-1 and MIC-4 at 1 µM, a concentration in the range of MC treatment, did not inhibit MMP-1 expression. Potentially, other phytoactives present in the MC conferred inhibitory effects on MMP-1. Further research will focus on identifying the phytoactives responsible for MMP-1 inhibition, as well as investigate additional mechanisms of action of the MC.
Conclusions
Solar radiation from UVA, UVB, visible and infrared rays increases the expression of MMP-1 in the skin and leads to inflammatory and oxidative damage.27 However, in the models presented, stable phytoactives isolated from moringa leaves were demonstrated to reduce such effects. MC therefore may act as a mitigating mixture of phytoactives, combating multiple targets involved in solar radiation-induced skin damage (see Table 1 and Figure 4). Based on the scientific data described herein, MC is believed to exhibit protective and restorative skin benefits which, when combined with its high stability and water solubility, make it an interesting candidate for protective as well as anti-aging skin compositions.
References
- www.klinegroup.com/news/global_natural_personal_care_market12-10-13.asp (Accessed Sep 22, 2014)
- JW Fahey, Moringa oleifera: A review of the medical evidence for its nutritional, therapeutic and prophylactic properties, Trees for Life Journal 1, 5 (2005)
- A Pandey et al, Moringa oleifera Lam. (Sahijan)—A plant with a plethora of diverse therapeutic benefits: An updated retrospection, Medicinal and Aromatic Plants 1, 1-8 (2012)
- F Anwar, S Latif, M Ashraf and AH Gilani, Moringa oleifera: A food plant with multiple medicinal uses, Phytother Res 21, 17-25 (2007)
- I Armand-Stussi, V Basocak, G Pauly and J McCaulley, Moringa oleifera: An interesting source of active ingredients for skin and hair care, SÖFW 129, 45-52 (2003)
- R Banerji, ABajpai and SC Verma, Oil and fatty acid diversity in genetically variable clones of Moringa oleifera from India, J Oleo Sci 58, 9-16 (2009)
- R Kleiman, DA Ashley and JH Brown, Comparison of two seed oils used in cosmetics, moringa and marula, Industrial Crops and Products 28, 361-364 (2008)
- J Tsaknis, S Lalas, V Gergis, V Dourtoglou and V Spiliotis, Characterization of Moringa oleifera variety Mbololo seed oil of Kenya, J Agric Food Chem 47, 4495-4499 (1999)
- www.treesforlife.org/our-work/our-initiatives/moringa/moringa-book (Accessed Sep 22, 2014)
- MC Palada, Moringa (Moringa oleifera Lam.): A versatile tree crop with horticultural potential in the subtropical United States, HortScience 31, 794-797 (1996)
- BS Rathi, SL Bodhankar and AM Baheti, Evaluation of aqueous leaves extract of Moringa oleifera Linn for wound healing in albino rats, Indian J Exp Biol 44, 898-901 (2006)
- A Caceres, O Cabrera, O Morales, P Mollinedo and P Mendia, Pharmacological properties of Moringa oleifera. 1: Preliminary screening for antimicrobial activity, J Ethnopharmacol 33, 213-216 (1991)
- SJ Franklin, SE Dickinson, KL Karlage, GT Bowden and PB Myrdal, Stability of sulforaphane for topical formulation, Drug Dev Ind Pharm 40, 494-502 (2014)
- YJ Surh, JK Kundu, and HK Na, Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals, Planta Med 74, 1526-1539 (2008)
- J Fahey, Y Zhangand P Talalay, Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens, PNAS 94, 10367-10372 (1997)
- AL Benedict, EV Knatkoand AT Dinkova-Kostova, The indirect antioxidant sulforaphane protects against thiopurine-mediated photooxidative stress, Carcinogenesis 33, 2457-2466 (2012)
- AT Dinkova-Kostova, JW Fahey, AL Benedict, SN Jenkins, L Ye, SL Wehage and P Talalay, Dietary glucoraphanin-rich broccoli sprout extracts protect against UV radiation-induced skin carcinogenesis in SKH-1 hairless mice, Photochem Photobiol Sci 9, 597-600 (2010)
- AT Dinkova-Kostova, JW Fahey, KL Wade, SN Jenkins, TA Shapiro, EJ Fuchs, ML Kerns and P Talalay, Induction of the phase 2 response in mouse and human skin by sulforaphane-containing broccoli sprout extracts, Cancer Epidemiol Biomarkers Prev 16, 847-851 (2007)
- C Waterman et al, Water extractable isothiocyanates from Moringa oleifera leaves attenuate inflammation in vitro, Phytochemistry 103, 114-122 (2014)
- TB Tumer, P Rojas-Silva, A Poulev, I Raskin and C Waterman, Direct and indirect antioxidant activity of polyphenol and isothiocyanate-enriched fractions from Moringa oleifera, Food Chemistry, in review (2014)
- AT Dinkova-Kostova and P Talalay, Direct and indirect antioxidant properties of inducers of cytoprotective proteins, Molecular Nutrition and Food Res 52, S128-S138 (2008)
- X Wu, GR Beecher, JM Holden, DB Haytowitz, SE Gebhardt and RL Prior, Lipophilic and hydrophilic antioxidant capacities of common foods in the United States, J Agric Food Chem 52, 4026-4037 (2004)
- S Grether-Beck, A Marini, T Jaenicke and J Krutmann, Photoprotection of human skin beyond ultraviolet radiation, Photodermatol Photoimmunol Photomed 30, 167-174 (2014)
- BA Halkier and J Gershenzon, Biology and biochemistry of glucosinolates, Annu Rev Plant Biol 57, 303-333 (2006)
- BL Graf et al, Quinoa phytochemicals inhibit matrix metalloproteinase and tyrosinase activity, Int J Cosmetic Sci, in review (2014)
- F Brugè, E Venditti, L Tiano, GP Littarru and E Damiani, Reference gene validation for qPCR on normoxia- and hypoxia-cultured human dermal fibroblasts exposed to UVA: Is β-actin a reliable normalizer for photoaging studies? J Biotechnol 156, 153-162 (2011)
- E Dupont, J Gomez and D Bilodeau, Beyond UV radiation: A skin under challenge, Int J Cosmetic Sci 35, 224-232 (2013)