Dandruff, a common scalp disorder, is considered to be a mild form of seborrhoeic dermatitis but without overt inflammation. It can lead to more severe seborrhoeic dermatitis, pityriasis versicolor and atopic dermatitis.1, 2 Dandruff has a multifactorial etiology, characterized by the lipophilic yeasts of the fungi genus Malassezia spp., sebum production and other factors that affect scalp health, such as environmental stress and hormonal changes.3 Consistent with the importance of Malassezia spp. in the etiology of dandruff is the fact that the most effective treatments for dandruff are antifungal agents, e.g., zinc pyrithione and ketoconazole that are usually applied as shampoos.4 Improvements in flaking also are highly correlated with reductions in the level of Malassezia spp. on the scalp.5
It is widely believed that increases in sebum production during puberty allow for the proliferation of Malassezia spp. on the scalp, especially M. globosa and M. restricta,1 since these entities use sebum lipids as a nutrient source. Secreted sebum consists of triglycerides and esters that are broken down by the dermal microbiota into diglycerides, monoglycerides and free fatty acids. Malassezia spp. produce lipases that hydrolyze triglycerides to generate specific saturated fatty acids, including oleic, that are used as nutrients and can also elicit a flaking response in dandruff-susceptible individuals.6 These free fatty acids play a key role in the initiation of the irritant response at the onset of dandruff.1
Malassezia spp. are commonly isolated from the openings of hair follicles—i.e., the follicular infundibulum, an area rich in sebum—and they rarely invade the skin.7 However, Malassezia spp. are members of the commensal skin microbiota readily isolated from the scalps of unaffected individuals.2 A number of studies have identified a 1.5–2.0 × increase in the numbers of Malassezia spp., especially M. globosa and M. restricta, on the scalps of patients with dandruff, compared with healthy controls.8, 9 Gathering evidence also points to the existence of pathogenic sub-types of M. globosa and M. restricta.10
Targeting Malassezia spp. as the causative agent in dandruff is key to any successful treatment, and current antifungal treatments are not wholly effective in situ regardless of their antifungal potential, due to formulation problems, lack of patient compliance and inter-individual differences in the efficacy of the various treatments available. Ideally, a novel agent targeting Malassezia spp. would be rapidly fungicidal, given the short contact time between hair treatments, e.g., shampoos and conditioners. It also would be water-soluble so that potentially irritating organic solvent-based formulations are not necessary and to make it easy to formulate into multiple product forms.
Membrane-active cationic antimicrobial agents such as a specific peptide tested here, referred to as NP108, have been found (in unpublished data) to interact with negatively charged microbial cell membranes—causing membranolysis and the loss of cellular contents, ultimately resulting in cell death. Also, since the membrane structure is governed by many different metabolic processes, cationic peptides are unlikely to engender acquired resistance in Malassezia spp., which is a key factor when considering topically applied antifungal agents. Modifications to several metabolic pathways would be required to alter membrane compositions sufficiently to confer resistance to membrane-active peptides/polymers.11
In this article, the authors examine the in vitro efficacy of a range of novel lysine polypeptides for antifungal activity against Malassezia spp. Lysine polypeptides were selected on the basis that ε-polylysine is generally recognized as a safe (GRAS) antimicrobial preservative; lysine polypeptides also are used in food products12 and may, therefore, be adaptable for cosmetics and personal care. GRAS substances are unlikely to engender adverse effects when applied to the skin, and cationic peptides are unlikely to penetrate the skin due to its high lipid content. However, appropriate testing to satisfy national and international regulatory bodies, e.g., the EU cosmetic products regulation EC No. 1223/2009, would be required.
To the authors’ knowledge, this is the first time antifungal polypeptides have been proposed to treat a dermal infection caused by Malassezia spp. After selecting candidate polypeptides showing significant in vitro efficacy, in vitro cytotoxicity and hematotoxicity were tested to confirm the safety profile of the polypeptides. The final candidate polypeptide, NP108, was then formulated into a novel vehicle and a variety of hair care products, which were subject to vitro efficacy testing to compare with conventional dandruff shampoos and conditioners. Finally, an in vivo murine M. pachydermatis skin infection model was used to determine the in vivo efficacy of NP108, compared with 2% w/v miconazole—an antifungal used clinically to treat yeast infections and applied in some anti-dandruff shampoos.
Materials and Methods
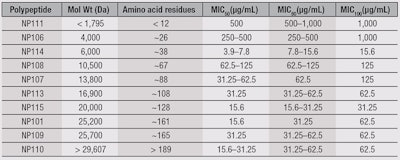
Peptides and media: The tested polypeptides were either produceda by solid-phase synthesis or purchasedb; the characteristics of the polypeptides, including molecular weights (Da) and amino acid residues, can be found in Table 1. All peptides were water-soluble. All other chemicals and growth mediac also were purchased, unless otherwise stated.
Test organisms: The minimum inhibitory concentration (MIC) of lysine polypeptides was determined versus M. pachydermatis CBS6536, M. pachydermatis NCPF3667 and M. furfur DSMZ6170 as model organisms by the broth microdilution method,13 substituting RPMI-1640 liquid medium with modified Christensen’s Medium (MCM) without agar. In brief, cells from a five day old culture were counted and diluted to 5 × 102 − 2.5 × 103 cells/mL (1 − 5 × 106 cells/mL for M. pachydermatis CBS6536) to improve reproducibility and consistency and exposed to serial dilutions of peptides in a 96-well plate.
In vitro protocols: Cultures were incubated at 35°C for 48 hr, and growth (optical density = 530 nm) was monitored at 0 hr and 48 hr. The MIC100 was the concentration at which no growth was seen. The MIC50 and MIC80 were the concentrations at which 50% and 80% of growth was inhibited. Hematotoxicity was determined against 10% human red blood cells according to the protocol of Lundin et al.14 Cytotoxicity was determined against human dermal fibroblast (BJ) and human lung epithelial (A549) cells using a viability/cytotoxicity kitd for mammalian cells according to the manufacturer’s instructions.
Peptide preparations: The polypeptide was prepared aseptically in a sterile PEG-14000 (PEG-14M) vehicle (65% w/v) with sterile deionized water (sdH2O). PEG-14M, 65% w/v, was specifically selected as it did not spread across the plate even when incubated at 30°C for up to five days. Zones of clearance, i.e., areas without fungi growth, could only result from the diffusion of NP108 from the vehicle. PEG-14M also is commonly used in the formulation of topical dermal drugs.
NP108 was added at 0.5% and 1.0% w/v, and a negative control of 65% w/v PEG-14M and sdH2O was used. All yeast inocula for this experiment were prepared to the 0.5 McFarland Standard.13 All experiments were carried out in triplicate with internal triplicate samples per experiment. Plates of modified Leeming Notman agar (MLNA) were prepared with 1.5% w/v agarose to limit inhibition of the migration/activity of NP108,15 and were inoculated with M. furfur DSMZ6170 or M. pachydermatis CBS6536. Antifungal agents in the PEG-14M vehicle were applied to the plates in discrete zones. Plates were incubated aerobically at 30°C for up to five days. Zones of clearance were recorded photographically.
Test product preparations: The antifungal efficacy of NP108 was then assessed in 10% v/v shampoo or conditioner vehicles. NP108 at a final 0.1% or 1.0% w/v concentration was formulated into a “normal,” non-anti-dandruff shampoo or conditioner, and also into a leading commercial anti-dandruff shampoo or conditioner to determine whether NP108 improved the antifungal efficacy of these products; no antifungal activity was demonstrated with either the shampoo or conditioner in the absence of NP108.
An additional control of Phosphate Buffered Saline (PBS) was used. All yeast inocula for this experiment were prepared to the 0.5 McFarland Standard. A 400-µL yeast inoculum sample was exposed to 100 µL of the materials described for up to 60 min at 37°C, followed by three washes with PBS to remove shampoo or conditioner. Serial dilutions were spread on MCM and incubated at 30°C for up to five days, and numbers of surviving colonies counted. All experiments were carried out in triplicate with triplicate samples. NP108 also was formulated at 0.5% w/v into a leave-in, light styling serum and a frequent use conditioner and tested for antifungal efficacy against M. pachydermatis CBS6536 and M. furfur DSMZ6170. M. pachydermatis was selected as M. furfur does not infect mice, and the other products were selected as possible vehicles for NP108 (although they were unsuccessful).
In vivo protocol: A study to determine the in vivo efficacy of NP108 polylysine also was commissioned; it employed a well-established murine model of M. pachydermatis seborrhoea. The clinical response (i.e., resolution of infection) was assessed to once-daily exposure to 5% w/v NP108, 2% w/v miconazole or vehicle of 65% w/v PEG-14M in H2O for six days 48 hr post-infection for six days, where n = 10 for each group. Mice were euthanized two days following the end of treatment.
Miconazole was selected as it is available commercially in a cream form, which unlike anti-dandruff shampoos, will remain in place on the mouse skin and not be readily lost. Clinical scores were assessed 10 days post-infection. To determine clinical scoring for skin colonization, skin samples were dissected into 10 pieces per study subject, and each piece was streaked onto MLNA for culture at 30°C for up to seven days. The number of quantifiable colony forming units (CFU) per streaked sample was read after three and seven days of incubation. Statistical analysis was conducted by Kruksal-Wallis all pairwise comparison and Fisher’s Exact test.
Results
The antimicrobial efficacy of the lysine polypeptides against M. pachydermatis CBS6536 was determined (see Figure 4). Here, low molecular weight polypeptides were least efficacious, whereas those with MW > 6 kDa were effective at ≥ 31.25 µg/mL. All peptides tested for hematotoxicity (NP101, 106, 107, 108, 109, 110 and 111) were not hemolytic at ≤ 10 mg/mL. NP113, NP114 and NP115 were hemolytic at concentrations < 10 mg/mL and were not used for further studies.
Peptides NP101 (25.2 kDa) and NP108 (17.6 kDa) were tested for cytotoxicity, and NP101 was more cytotoxic—with an LD50 = 2,700 µg/mL for BJ, and = 210 µg/mL for A549—than NP108, with an LD50 = 4,500 µg/mL for BJ, and 15,000 µg/mL for A549. Therefore, NP108 was selected for further development.
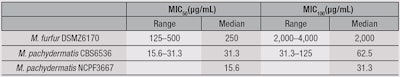
NP108 was then tested for antimicrobial efficacy versus M. furfur DSMZ6170, M. pachydermatis NCPF3667 and M. pachydermatis CBS6536 (see Table 2). The MIC values obtained for NP108, particularly against M. pachydermatis (MIC100 = 31.3-125 µg/mL), were therapeutically promising, but since in vitro MICs of non-formulated active ingredients are generally not reflective of their activity in situ or in final formulations such as shampoos and conditioners, their in vivo activity was further assessed under more physiologically and relevant conditions to determine their potential.
To test the compatibility of NP108 with existing anti-dandruff shampoos and conditioners, NP108 was incorporated into commercially available formulations; their antimicrobial efficacy was assessed against M. pachydermatis CBS6536 and M. furfur DSMZ6170. Results of the conditioners tested indicate both strains of Malassezia spp. were killed, and as such, NP108 is considered compatible for inclusion in existing dandruff product ranges. In order to maximize contact time, it would be most suitable to incorporate NP108 into a frequent use conditioner or other scalp treatment, rather than a shampoo with a limited contact time of typically< 3 min.
Figure 1a-b demonstrate that only 3 min of exposure to a hair conditioner containing 1.0% w/v NP108 eradicated or at least significantly reduced the number of recoverable CFU of M. pachydermatis CNS6536 and M. furfur DSMZ6170, respectively. Three minutes is a reasonable contact time to assume for the application of conditioning products to the hair/scalp. A conditioner containing 0.04% w/v zinc pyrithione—a concentration found in several commercially available dandruff products—was compared in this experiment and found to be less effective than NP108. The effect of NP108 was statistically significant using one-way ANOVA, compared with the control at all time points (p = 0.0008). Specifically, 3 min of exposure to zinc pyrithione eradicated no significant amounts of M. furfur DSMZ6170. Similar results demonstrating the efficacy of NP108’s over zinc pyrithione were observed for shampoo formulations (data not shown).
Malassezia spp. is associated with dermal infections other than dandruff, including pityriasis versicolor, seborrhoeic dermatitis and atopic dermatitis.1, 2 Therefore, NP108 was again formulated into a 65% w/v PEG-14M vehicle suitable for application to the skin, and tested for its antimicrobial efficacy against M. pachydermatis CBS6536. Again, the 65% w/v PEG-14M vehicle was chosen since it did not spread across the surface of an agar plate for 72 hr at 37°C, and it is a cosmetically attractive gel-like vehicle. Figure 2 demonstrates that 0.5% and 1.0% w/v NP108 could kill M. pachydermatis CBS6536, creating zones of clearance after application to 1.5% w/v agarose plates.
While the authors already demonstrated that NP108 was compatible with marketed dandruff products, they also investigated whether it was possible to create bespoke hair care formulations around NP108. To that end, 0.5% w/v NP108 was formulated into a number of products, including a leave-in serum for the scalp and hair and a frequent-use conditioner. These products were tested for antifungal efficacy against M. pachydermatis CBS6536 and M. furfur DSMZ6170.
Figure 3 shows the efficacy of NP108 versus M. pachydermatis CBS6536 in the frequent use conditioner and leave-in serum—note that the contact time for a leave-in serum is significantly longer than for a conditioner and is, therefore, likely to improve antimicrobial efficacy. Similar effects were observed when used against M. furfur DSM6170 (data not shown).
Finally, 5% w/v NP108 in the 65% w/v PEG-14M vehicle was tested in vivo to determine its antimicrobial efficacy and safety in a murine model of M. pachydermatis skin infection, in comparison with the antifungal miconazole at 2% w/v, which is the active agent in medicated anti-dandruff shampoos.
NP108 at 5% w/v, applied over a six-day period, significantly reduced the severity of an established M. pachydermatis infection (see Figure 4), based on the number of CFU obtained from skin biopsies. The group average was 34.6 CFU/mouse at p = 0.0004. NP108 also reduced the clinical scores of infections overall, i.e., changes in skin color and texture (p < 0.0001), versus scores in control groups and compared with vehicle-only controls. The control group average was 135.8 CFU/mouse, and the miconazole treatment group average was 131.4 CFU/mouse. Unlike NP108, the clinical scores began to increase, i.e., worsen, after the treatment period ended (see Figure 4). Treatment with the test article (NP108) was well-tolerated throughout the study.
Discussion and Conclusions
The results of the described studies indicate that a specific membrane-active lysine polypeptide, NP108, has significant potential as a safe and efficacious antifungal agent potentially for the treatment of dermal Malassezia spp. infections and dandruff. NP108 was rapidly effective against Malassezia spp. when formulated into a simple PEG-14M vehicle and importantly, when incorporated into commercially available and bespoke shampoo, conditioning and scalp treatment (serum) formulations, without a loss of activity, as compared with non-formulated NP108.
Finally, when tested in vivo in a murine model of M. pachydermatis infection, NP108 was well-tolerated and significantly reduced all infection parameters assessed. It also out-performed a commercially available antifungal, 2.0% w/v miconazole. Human and murine skin are obviously distinct in structure and composition of sebum and fatty acids, but this provides promising preclinical data in a complex biological system, which adds to the evidence presented for the application of polylysine peptide-based treatments for dandruff.
References
- TL Dawson, Malassezia globosa and restricta: Breakthrough understanding of the etiology and treatment of dandruff and seborrheic dermatitis through whole-genome analysis, J Invest Derm Symp Proc 12(2) 15–19 (2007)
- HK Park, M-H Ha, S-G Park, MN Kim, BJ Kim and W Kim, Characterization of the fungal microbiota (mycobiome) in healthy and dandruff-afflicted scalps, PLoS One 7(2) e32847 (2012)
- GA Turner, M Hoptroff and CR Harding, Stratum corneum dysfunction in dandruff, Int J Cosmet Sci 34(4) 298–306 (2012)
- GE Piérard, JE Arrese, C Piérard-Franchimont and RP De Doncker, Prolonged effect of antidandruff shampoos—Time to recurrence of Malassezia ovalis colonization, Int J Cosmet Sci 19(3) 111–117 (1997)
- JR Schwartz, CM Cardin and TL Dawson, Dandruff and seborrheic dermatitis, in Textbook of Cosmetic Dermatology, R Baran and HI Maibach, eds, Martin Dunitz Ltd, London, UK (2004) pp 259–272
- BI Ro and TL Dawson, The role of sebaceous gland activity and scalp microfloral metabolism in the etiology of seborrheic dermatitis and dandruff, J Invest Dermatol Symp Proc 10(3) 194–197 (2005)
- RJ Hay, Malassezia, dandruff and seborrhoeic dermatitis: An overview, Brit J Dermatol 165(S2) 2–8 (2011)
- KJ McGinley, JJ Leyden, RR Marples and AM Kligman, Quantitative microbiology of the scalp in non-dandruff, dandruff and seborrhoeic dermatitis, J Invest Derm 64(6) 401–405 (1975)
- CM Gemmer, YM DeAngelis, B Theelen, T Boekhout and TL Dawson, Fast, noninvasive method for molecular detection and differentiation of Malassezia yeast species on human skin and application of the method to dandruff microbiology, J Clin Microbiol 40(9) 3350–3357 (2002)
- M Tajima, T Sugita, A Nishokawa and R Tsuboi, Molecular analysis of Malassezia in seborrheic dermatitis patients: Comparison with other diseases and healthy subjects, J Invest Dermat 128(2) 345–351 (2008)
- DK Mercer and DA O’Neil, Peptides as the next generation of anti-infectives, Future Medicinal Chemistry 5 315–337 (2013)
- S Shima, H Matsuoka, T Iwamoto and H Sakai, Antimicrobial action of epsilon-poly-L-lysine, J Antibiot (Tokyo) 37(11) 1449–1455 (1984)
- Clinical and Laboratory Standards Institute (CLSI), Reference Method for broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved Standard–Third Edition, CLSI document M27–A3, Wayne, PA (2007)
- P Lundin, SEL Andaloussi and U Langel, Toxicity methods for CPPs, Methods in Molecular Biology 683 195–205 (2011)
- RI Lehrer, M Rosenman, SS Harwig, R Jackson and P Eisenhauer, Ultrasensitive assays for endogenous antimicrobial polypeptides, J Immunol Methods 137(2) 167–173 (1991)