The story of sunscreen photostability is really the story of how sunscreens work. The principal characters are photons and electrons. Their stages are tiny, and the acts in their dramas are extremely short-lived. Take, for example, a UVB photon with a wavelength of 300 nm (3000 Å). If one imagines a photon as a round object and its wavelength as its diameter, one can calculate the time it takes for it to pass a point in space by dividing its diameter, 3 x 103 Å, by its speed—the speed of light, c, or 3 x 1018 Å/sec. Thus the short journey of a 300-nm photon takes just 10-15 sec, a single femtosecond (i.e., one one-thousandth of a trillionth of a second), to complete.
Now take the interaction of a photon with a single electron, currently occupying a non-bonding (n) or π (pi) orbital in an organic molecule. The electron’s first role is to use all the energy in the photon to jump to a higher energy orbital. This initial transition to a higher energy orbital takes the molecule from the ground state to its first excited state, known as the singlet excited state. By doing so, it makes the photon completely disappear but it must act in a very short time since, as noted, it only takes the photon 10-15 sec to pass. If successful, the electron’s role quickly shifts to one that appears much less scripted and more improvisational; for example, the singlet excited state often transitions to the triplet excited state. Of course, each photon-absorbing electron is playing a small role in the larger drama performed by the sunscreen: that of protecting the wearer’s skin from UV radiation.
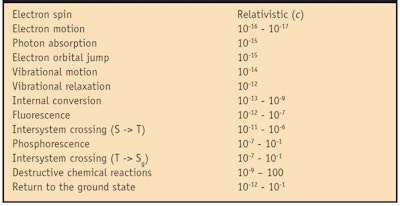
Table 1 lists typical time scales for the dynamic process that sunscreen active ingredients, i.e., UV filters, undergo, starting with electron motion and ending, if all goes well, with its unchanged return to a “ready-to-absorb-another-photon” state, known as the ground state. Suffice it to say that the sooner this happens, the less likely something will occur to prevent it. A good part of the sunscreen formulator’s job is to understand this concept and to employ appropriate means to encourage UV filters in formulations to return rapidly to the ground state.
The transfer of energy from a photon to an electron must take place in the brief time that the photon and the electron share the same space, which as stated is roughly 10-15 sec. There is, however, a further condition that must be met before the energy transfer can take place: the resonance condition. Photochemists visualize the electron as moving from the highest occupied (HO) molecular orbital to the lowest unoccupied (LU) molecular orbital, with the further proviso that the energy difference between HO and LU must exactly match the photon’s energy. The photon’s energy is expressed as hν, where h is Planck’s Constant (6.626 x 10-34 J sec) and the Greek letter ν (nu) is the photon’s frequency. When the energy levels match, the resonance condition is met and the energy transfer can occur.
However, even a cursory examination of Table 1 reveals the inescapable fact that while some dynamic processes take place on shorter time scales (i.e., electron motion or vibrational motion), other processes have time scales that overlap one another (i.e., internal conversion and fluorescence). This correctly implies that there is a competition between dynamic processes, much like a race, to determine which path or paths the excited electron will take to deactivation. This race is reflected in the rate at which things happen-and knowing the rates of each of the competitive processes allows the photochemist to handicap the race by predicting which process, processes or outcomes are likely to prevail under a given set of circumstances. Sunscreen formulators can do this too, and thereby give themselves and their formulations the best chance to meet desired sun protection goals. Figure 1 puts these dynamic processes in context and some semblance of order, for the reader’s reference.
In the excited state, the UV filter molecule has acquired excess energy that it must dispose of in order to return to the ground state. To aid in this process, formulators have added to their sunscreens certain compounds such as octocrylene (OC), which has the ability to limit or quench the UV filter’s triplet excited state, returning it to the ground state and reducing or eliminating destructive chemical reactions. As noted, oftentimes a molecule in the singlet excited state transitions to the triplet excited state. In practice, all destructive chemical reactions begin when UV filter molecules are in the triplet excited state. Compounds possessing this quenching ability are known to sunscreen formulators as photostabilizers or triplet quenchers. OC, among a few other compounds, is effective at high concentrations but less effective at low concentrations and when particularly reactive UV filters such as avobenzone and octyl methoxycinnamate (OMC) are combined.
Recently, a new photostabilizer was developed, ethylhexyl methoxycrylenea, which quenches the singlet excited state of UV filters (see Figure 1). A photostabilizer that quenches the singlet excited state reduces the number of molecules that enter the triplet excited state, thus further reducing the possibilities for destructive photochemical reactions. Theoretically, a singlet quencher such as ethylhexyl methoxycrylene would be effective at lower concentrations than a triplet quencher such as OC in all formulations and would improve the performance of formulations that are difficult for triplet quenchers to photostabilize, such as those that combine avobenzone and OMC.
Qualitative means were used to test ethylhexyl methoxycrylene’s ability to quench the visible fluorescence of avobenzone, OMC and other fluorescing compounds. Sophisticated means were used to measure avobenzone’s fluorescence—spectra, quantum yield, and lifetime—and to compare ethylhexyl methoxycrylene and OC for their abilities to operate on avobenzone’s singlet excited state. Several in vitro performance and dose response studies also were conducted by irradiating thin films containing varying concentrations of methoxycrylene with avobenzone alone and with other photoactive compounds. Finally, creams and lotions were prepared using ethylhexyl methoxycrylene and avobenzone and other UV filters, and clinical studies were performed to determine both the SPF and UVA protection factors.
Materials and Methods
Avobenzone and OCb were obtained for this study, as well as OMCc and ethylhexyl methoxycrylenea. The solvents used were spectrophotometric grade, and the materials used to prepare creams and lotions were acquired as samples from commercial sources. Fluorescence spectra were measured on a spectrofluorimeterd. The avobenzone and OMC were dissolved either in ethanol or ethyl acetate to an apparent concentration of ~2 mg/L. The final concentrations were adjusted to give an absorbance of ~0.15 in a 1 cm cuvette. These solutions were placed in 1 cm fluorescence cuvettes, inserted into the instrument, and scanned under “Matrix scan conditions” to identify optimal excitation (varying lex from 200 nm to 350 nm) and emission wavelengths (scanned from 300 nm to 800 nm) in the UV region. Spectra are reported from 300 to 500 nm to avoid the impression of false fluorescence from second order diffraction.
Resolutions used were 0.2 nm and time constants of 1 sec. Under these conditions, it was relatively simple to detect the Raman signals from the solvents used. The baselines (solvent fluorescence and Raman) were subtracted from the sample spectra. Quantum yield of fluorescence was determined by the spectrofluorimeter using diphenyl anthracene (DPA) as the standard.
Qualitative fluorescence quenching experiments were conducted by spotting solutions on thin layer chromatography plates and illuminating them with long wave (peak 365 nm) UVR, then photographing theme. Solutions were prepared by dissolving avobenzone or OMC and either potential quencher or non-photoactive diluent in ethyl acetate.
Fluorescence lifetimes and quenching measurements were determined on a streak scopef. Fluorescence lifetime data were measured by exciting the samples using pulses centered at 400 nm, derived from the frequency doubled output of a 40 kHz regeneratively amplified titanium sapphire laser system with a 150 fs pulse width. The spectra and time decay remained unchanged at fluences up to 200 μJ/cm2. The fluorescence was collected using a 20 cm collecting lens and focused onto the slit of the streak camera. IR and bandpass filters were used to block any residual 800 nm and 400 nm light from reaching the detector.
Solutions for fluorescence lifetime and quenching measurements were made using avobenzone, octocrylene and ethylhexyl methoxycrylene in ethanol and ethyl acetate. All solutions had an optical density of < 0.1 at the excitation maximum to eliminate self-absorption. Solutions for the quenching experiments were made at the following concentrations: avobenzone at 0.4 mM and octocrylene at 30 mM; and avobenzone at 0.4 mM and ethylhexyl methoxycrylene at 0–18 mM. Solutions were placed in a 1 cm path length quartz cuvette.
In vitro performance and dose response measurements were made on a transmittance analyzerg. PMMA platesh and Vitro-skinj were the substrates. Solutions were prepared in ethyl acetate and included 2% octadecene/MA copolymer as a film former, as well as varying amounts of a non-photoactive diluent in order to equalize the volumes of oil deposited on the substrate. UVR was provided by a solar simulatork. Clinical SPF and UVA protection factors were determined independentlym using FDA and JCIA (PPD) protocols, respectively.
Results
Fluorescence in ethyl acetate: When excited at 330 nm in ethyl acetate, avobenzone fluoresced with a peak of approximately 405 nm and a visible tail out past 500 nm. When excited at 310 nm in ethyl acetate, OMC fluoresced with a peak of approximately 368 nm and a visible tail out to about 440 nm (see Figure 2a-b).
Quantum yields of fluorescence: The quantum yield is the ratio of the number of molecules that emit a photon from the singlet excited state to the number of molecules that absorb a photon. Using diphenyl anthracene (DPA) as standard, at an excitation of 330 nm, the quantum yield of fluorescence for avobenzone in ethyl acetate was 0.0104; in other words, out of 100 avobenzone molecules excited by a photon, only about one fluoresces (i.e., emits a photon from the singlet excited state).
Qualitative fluorescence-quenching experiments: Ethylhexyl methoxycrylene was found to quench the visible fluorescence of avobenzone, whereas octocrylene did not (see Figure 3a-b); and compared with OC, ethylhexyl methoxycrylene quenched the visible fluorescence of OMC much more strongly (data not shown).
Fluorescence lifetime and quenching experiments: The fluorescence lifetime of avobenzone was determined to be 1.3 x 10-11 seconds (13 picoseconds) in ethanol. Ethylhexyl methoxycrylene was found to quench avobenzone’s singlet excited state in a manner relevant to its concentration and not in a linear way (see Figure 4). In contrast, octocrylene did not quench avobenzone’s singlet excited state, even at a 75:1 molar ratio of octocrylene to avobenzone (see Figure 5).
In vitro photostability experiments: A thin film made from a solution containing 3% avobenzone, 7.5% OMC and no photostabilizer retained only 44.5% of its UVA absorbance after 25 MED. With 3% ethylhexyl methoxycrylene (3% OC), the film retained 83.7% (53.9%) of its UVA absorbance after 25 MED. With 5% ethylhexyl methoxycrylene (5% OC) the thin film retained 89.8% (63.7%) of its UVA absorbance (see Figure 6). When compared with octocrylene, to photostabilize a thin film made from a solution containing 3% avobenzone and no OMC, ethylhexyl methoxycrylene again provided superior performance, albeit by a smaller margin (see Figure 7).
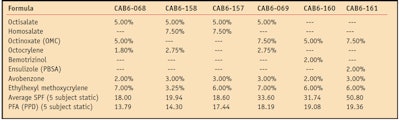
After 25 MED, the thin film photostabilized by 3% ethylhexyl methoxycrylene retained 90.4% of its UVA absorbance and 97.7% of its UVB absorbance. Photostabilized by 3% octocrylene, the thin film retained 85% of its UVA absorbance and 92.9% of its UVB absorbance. A sunscreen formulation containing 7.5% OMC, 3% avobenzone, 2% ensulizole (PBSA) and 6% ethylhexyl methoxycrylene achieved SPF 50.8 and PFA 19.36 in vivo. Table 2 shows the results of clinical testing on sunscreens that contain ethylhexyl methoxycrylene. Note that all formulations tested easily met the current EU recommendation that the SPF:PFA ratio be no greater than 3.
Discussion
Sunscreens that contain organic UV filters are photochemical systems. One key to understanding how they work, and how to coax them to work better, is to know the time scales at which events within them occur. The initial event—the absorption of a photon and elevation of an electron to a higher energy orbital—takes place in about 10-15 seconds, as noted, which is also approximately the time it takes for a photon of 300 nm to pass a point in space. To put this into perspective, a little over 1015 seconds ago, dinosaurs roamed the earth.
The timescales for subsequent events vary by compound and experimental conditions. For avobenzone, the deactivation of its singlet excited state by internal conversion, fluorescence, solvent interactions or intersystem crossing to the triplet state takes place very quickly, within 1.3 x 10-11 seconds (13 picoseconds) in ethanol, as measured by the streak scope experiments. In contrast to the brief lifetime of the singlet excited state, the lifetime of the triplet excited state is on the order of 4 x 10-7 seconds (0.4 microseconds),1 or about four orders of magnitude greater. And the transient isomeric structures produced from the triplet excited state may persist for much longer (milliseconds), depending on the solvent.2
Those with a formal background in chemistry will know that kinetics is the study of reaction rates, the difference here being that, at a molecular level, the initial “reaction” is the absorbance of a photon and simultaneous excitation of an electron. The kinetics of photon absorption is determined solely by the probability that a UV filter molecule in its ground state will encounter a photon that meets the resonance condition. Once absorption/excitation takes place, the rate at which the UV filter molecule continues to absorb photons depends additionally on how long it takes for the molecule to dissipate its excited state energy and return unchanged to the ground state. The key word is unchanged, because even the slightest change to the molecule’s structure or geometry can irrevocably destroy its UV photon absorbing ability.
To calculate the rates of various deactivation processes, the quantum yield of the process is divided by its lifetime: k = φ / τ . So, to calculate the rate of fluorescence kf , divide the quantum yield of fluorescence Φf (0.01) by its fluorescence lifetime τf (1.3 x 10-11). This gives a radiative rate from the singlet excited state of 7.69 x 108 sec-1. Similarly, the non-radiative rate, which is the sum of all the other physical and chemical deactivation processes, can be calculated by dividing the quantum yield Φnr (0.99) by the singlet lifetime τS1 (also 1.3 x 10-11). This gives a non-radiative rate of 7.62 x 1010 sec-1 or about 76 ns-1, a rate that is almost two orders of magnitude greater.
Clearly non-radiative (internal conversion, intersystem crossing, quenching) processes dominate the deactivation of avobenzone’s singlet excited state. Fluorescence almost seems like an afterthought, albeit a useful one. As the qualitative fluorescent spots experiments indicated, and the streak scope experiments confirmed, ethylhexyl methoxycrylene has the ability to quench avobenzone fluorescence—a quality that its close chemical relative, octocrylene, does not share. The extremely short singlet excited state lifetime of avobenzone, 13 picoseconds (thirteen one thousandths of a billionth of a second), makes this quality of ethylhexyl methoxycrylene all the more surprising.
Intuitively, the faster a photostabilizer works, i.e., the faster it brings its photonically excited and less stable target to the more stable ground state, the fewer opportunities there are for destructive chemical reactions and the more effective it will be. In the case of ethylhexyl methoxycrylene, intuition is born out. As the data shows, ethylhexyl methoxycrylene sets a new standard for photostabilization of avobenzone, even when avobenzone is combined with OMC.
Conclusions
Avobenzone’s singlet excited state is extremely short-lived at 13 picoseconds. Even so, ethylhexyl methoxycrylene quenches avobenzone’s singlet excited state in a concentration-related manner. At equal weight concentrations, ethylhexyl methoxycrylene was found to be more effective than octocrylene at preserving avobenzone’s UVA and UVB absorbance in the absence of OMC. In addition, ethylhexyl methoxycrylene is superior to octocrylene at preserving avobenzone’s UVA and UVB absorbance in the presence of OMC. Finally, formulations containing avobenzone and OMC can achieve high UVA protection factors when photostabilized with ethylhexyl methoxycrylene.
What does this mean for sunscreen formulators? In two words: greater efficiency. It all boils down to energy. Sunscreens protect the skin from the sun’s energy by intercepting solar UV radiation, and ethylhexyl methoxycrylene protects the active ingredients in sunscreens by improving their ability to dissipate the energy that they have intercepted—and it does it faster than any other photostabilizer currently used.
By incorporating ethylhexyl methoxycrylene in sunscreens, formulators can achieve higher levels of performance from the same or lower levels of active ingredients. This is true across the solar UV spectrum, from 290 to 400 nm, but it is especially true in the UVA portion of the spectrum, 320–400 nm, where formulators have traditionally had difficulty meeting protection goals.
Recently, UVA protection goals have become more formalized in Europe, and have been given the force of regulation in Japan.3,4 In the United States, new rules have been proposed that even the FDA admits will be difficult for sunscreen formulators to achieve.5 These proposals, guidelines and rules are driving much of the sunscreen formulating around the world today. Ethylhexyl methoxycrylene gives formulators a new tool that makes meeting the new standards much less difficult to achieve.
Acknowledgments: The authors gratefully acknowledge the contributions of Tim Keiderling of the University of Illinois-Chicago. Major elements of this article were presented at the IFSCC held in Melbourne, Australia, in October 2009 and at the SCC Scientific Meeting held in New York City in December 2009.
References
- E Chatelain and B Gabard, Photostabilization of butyl methoxydibenzoylmethane (avobenzone) and ethylhexyl methoxycinnamate by bis-thylhexyloxyphenol methoxypheyl triazone (Tinosorb S), A new UV broadband filter, Photochem Photobiol 74(3) 401-406 (2001)
- A Aspee, C Alliaga and JC Scaiano, Transient enol isomers of dibenoylmethane and avobenzone as efficient hydrogen donors toward a nitroxide pre-fluorescent probe, Photochem Photobiol 83 481-485 (2007)
- Commission recommendation on the efficacy of sunscreen products and the claims relating thereto, Official Journal of the European Union, L 265/39 (Sep 26, 2006)
- JCIA measurement standard for UVA protection efficacy, Japan Cosmetic Industry Association (JCIA) 9-14, Toranomon 2-Chome, Minato-Ku Tokyo, 105 (1995)
- Sunscreen Drug Products for Over-the-Counter Human Use: Proposed Amendment of Final Monograph, Federal Register, 72 (165) (Aug 27, 2007)