Abstract: Quaternary ammonium compounds have raised eco and health concerns, driving demand for alternatives. Here the clinical efficacy of cationic emulsifier-based quat-free solutions to provide lasting moisture was compared with that of a quat-based benchmark.
Dry skin (xerosis cutis) is a prevalent condition characterized by itchy, dry, rough patches that can cause discomfort and reduce overall skin health. Causes vary and include extrinsic factors such as climate, cleansing and occupational exposure, and intrinsic factors such as skin disorders, systemic diseases, psychiatric conditions, diet and medications. 1
Topical treatments such as therapeutic lotions can relieve symptoms of dry skin, restore moisture and improve skin’s moisture barrier.1 These lotions often include quaternary ammonium compounds (QACs), which have been used as moisturizing and conditioning ingredients in skin and hair care for decades.
For example, the first use of QACs in skin care dates back to 1951 with the launch of Baby Magic productsa.2 Kao launched Curél, its first cationic lotion containing a QAC, in 1984, and products containing QACs remain popular to this day.3
In terms of performance, QACs are ideal ingredients for minimizing greasiness, imparting a soft, powdery feel, and stabilizing high oil loads in a moisturizing lotion.4 Additionally, QACs deliver therapeutic benefits and clinically proven moisturization, with the lamellar gel networks formed by QACs further enhancing sensorial properties, improving the skin barrier function, imparting occlusivity and providing scaffolding for keratinocytes.4-6
Countering these advantages, the use of QACs has been associated with risks to human health and aquatic life, along with environmental and regulatory concerns.7-9 This is because, due to their permanent cationic charge, QACs have a strong affinity for binding to anionic surfaces and can accumulate in the environment, adhering to sediments and persisting in waterways despite water treatment.9
In humans, QACs have been linked to skin-related issues such as dermatitis, and also have been associated with respiratory, developmental, reproductive and metabolic concerns.7, 8 The regulatory landscape surrounding the use of QACs is evolving to mitigate these risks.
There is thus a need for alternative ingredients to QACs to circumvent these environmental and health concerns while delivering comparable performance and biodegradability. Similarly, there is currently a gap in the market particularly for therapeutic cationic lotions with high naturality that are safe for humans and the environment.
To address these needs, in the present work, quat-free cationic lotions were formulated and tested in a 24-hr clinical study to evaluate their moisturization efficacy. Results were compared with a commercial benchmark lotion containing a quaternary ammonium compound.
Experimental Design and Methods
Clinical study design: A double-blind clinical study was conducted to evaluate skin moisturization. Three cationic lotions were evaluated on the subjects’ lower legs over a 24-hr period following application. Prior to initiation of the study, the proposed protocol, Informed Consent Form (ICF) and product information were submitted to the Allendale Investigational Review Board for approval, which upon review was granted.
Thirty female subjects, ages 25 to 55 years old, were enrolled in the study with a minimum of six subjects per Fitzpatrick skin phototype pairing (I-II, III-IV, V-VI). Qualifying subjects had dry skin – more specifically, an average baseline skin hydration valueb of < 40 a.u. was mandatory for participation in the study. Subjects agreed not to alter/tamper with test sites, including not using exfoliating or topical products and avoiding sun exposure three days prior to the study.
On the day of the study, subjects refrained from showering, avoided participating in activities that increased body temperature, and wore clothing that allowed access to test sites. Subjects were excluded from the study if they had any condition that could impact the results; e.g., known allergies or sensitivities to key ingredients (oats, cocamidopropyl betaine, etc.); were pregnant, breast-feeding or planning pregnancy during the study; undergoing menopause; participating in another clinical study; or had damaged skin around the test sites (sunburn, uneven skin tone, tattoos, scars, etc.).
Test protocol: Three days prior to the test, subjects were instructed to use Ivoryc bar soap twice daily to facilitate dryness. On the day of the study, subjects acclimated in an environmentally controlled room (ECR) and their baseline skin hydration measurements were takenb using skin capacitance.10 A technician then applied 0.05 cm3 of each of three cationic lotions (Lotion A, B and C) in a randomized fashion to the respective test sites, leaving one untreated site (no Rx) as a control.
Subjects returned to the ECR at designated time points (15 min, 8 hr and 24 hr) following application of the lotions for additional hydration measurements. At each timepoint, subjects acclimated for a minimum of 25 min before measurements were taken. Five measurements were taken for each test site and were averaged for analysis. Statistical analyses were performed using a two-tailed t-test with a significance level of p < 0.05.
Test Materials
Cationic lotions: As noted, three cationic lotions were evaluated during this study. Lotion A used the protonated tertiary amine brassicamidopropyl dimethylamine as the cationic alternative (see Figure 1a). Lotion B contained the primary amino lipid brassicyl valinate esylate (BVE) as the cationic alternative (see Figure 1b).
Finally, Lotion C, the control composition, was based on a market-leading cationic lamellar lotion with known clinical moisturization benefits. Lotion C contained the QAC distearyldimonium chloride (see Figure 1c).
Lotion A was designed to be a 1:1 match to the benchmark, with the inclusion of the quat-free cationic emulsifier in Lotion A versus the QAC emulsifier of Lotion C being the only difference. Lotion B was designed to be a 100% natural alternative to Lotion C. Notably, to exist in their cationic state, it was crucial for lotions A and B have a pH of < 7 to ensure effective performance.
Formulation ingredients: Humectants, emollients and occlusive agents were incorporated into the lotions to ensure effective moisturization, with each lotion containing identical percentages of glycerin and colloidal oatmeal. Additionally, the three lotions contained functional ingredients such as pH adjusters, stabilizers and preservation systems. Ingredients and pH values for the lotions are reported in Table 1.
Results
Thirty subjects participated in the clinical study, all of whom completed it with no adverse reactions. Fitzpatrick skin phototypes were evenly distributed among the subjects: 10 subjects for types I-II; 9 subjects for types III-IV; and 11 subjects for types V-VI.
Figure 2 shows the mean changes in capacitance values from the baseline measurements at 15 min, 8 hr and 24 hr after application (or not – no Rx) of the lotions to the test areas. The moisturizing effect was highest at the 15-min timepoint, with all three cationic lotions exhibiting a statistically significant (p < 0.001) increase in skin capacitance from the baseline measurement, consistent with immediate moisture replenishment.
The values of the capacitance measurements were lower for all three lotions at the 8-hr and 24-hr timepoints, as compared to the 15-min timepoint, but higher than the baseline measurements, indicating the moisturization benefit lasted 24 hr for all three lotions (p < 0.001) but lessened with time.
The untreated control sites did not exhibit significant differences in capacitance from the baseline measurements at 15 min and 8 hr, although a significant difference was observed at 24 hr (p < 0.001). This could potentially be due to the discontinuation of using the Ivory bar soap, the skin naturally replenishing its moisture, and/or the product spreading from adjacent sites.
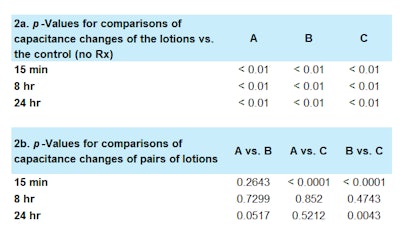
Additional statistical analyses are found in Table 2. Comparisons of capacitance changes of the lotions versus those of the control were statistically significant at all timepoints (p < 0.01; see Table 2a), including at 24 hr. When comparing Lotion B and Lotion C, a statistically significant larger increase in capacitance versus the baseline was observed for Lotion B at the 15-min (p < 0.0001) and 24-hr (p < 0.01) timepoints (see Table 2b).
Compared with Lotion C, sites treated with Lotion A also exhibited a statistically significant greater increase in capacitance at 15 min (p < 0.0001) but no significant differences were observed at 8 hr or 24 hr (see Table 2b). Similarly, no significant differences were found in comparisons of any of the other treated sites at any timepoints (p > 0.05 for all; see Table 2b).
Discussion
Most therapeutic cationic lotions available in the marketplace and backed by clinical data contain QACs. As concerns regarding the potential health and environmental issues surrounding the use of QACs grow, designing safe and effective quat-free cationic lotions, including alternatives with high naturality, is vital.8, 9 This need was addressed by the present double-blind clinical study of three cationic lotions, with the moisturization efficacy monitored over a 24-hr period. Hydration was measured through capacitance measurements – water has a high dielectric constant, so increased capacitance indicated higher hydration of the stratum corneum.10
The formulations of Lotion A and Lotion B incorporated cationic QAC alternatives that are readily biodegradable and non-toxic to humans and the environment.11 Lotion A contained the tertiary amine brassicamidopropyl dimethylamine (see Figure 1a). This plant-based amine is produced according to the Principles of Green Chemistry, with its lipophilic alkyl tail derived from the renewable feedstock Brassica campestris (rapeseed) oil.11-13 It has a biobased content of 83% and exists in its protonated form at the pH of Lotion A (6.50).
Lotion B, a 100% natural cationic lotion, incorporated the primary amino lipid BVE as the cationic alternative (see Figure 1b). This amino lipid is 100% biobased with the cationic ammonium head group derived from the amino acid L-valine and the alkyl tail formed from vegetable fatty alcohols.11, 14-15 BVE has a biodegradable ester linkage and exists in its protonated form at the pH of Lotion B (4.00). It is produced in the absence of solvents in a green process, with water as the only by-product.15
The moisturization efficacies of Lotion A and Lotion B were compared with the performance of Lotion C, which contained distearyldimonium chloride, a QAC (see Figure 1c). The composition of Lotion C was based on a commercial daily moisturizing lotion with known clinical moisturization benefits.16 Lotions A and C contained isopropyl palmitate, petrolatum and dimethicone. Although these ingredients are known for their moisturizing performance and sensory enhancement in lotions, they are not 100% natural.
In Lotion B, these petro-derived ingredients were replaced with 100% natural alternatives. Natural ingredients with sensory profiles similar to those of the corresponding petro-derived ingredients were selected, with: heptyl undecylenate replacing isopropyl palmitate; caprylic/capric triglyceride (and) hydrogenated rapeseed oil replacing petrolatum; and diheptyl succinate (and) capryloyl glycerin/sebacic acid copolymer replacing dimethicone.
The results of the present clinical study indicated that quat-free Lotions A and B provided greater immediate moisturization at the 15-min timepoint and comparable moisturization at 24 hr as compared to Lotion C (see Figure 2). No significant differences in moisturization efficacy were observed between quat-free Lotions A and B (see Table 2b). Overall, the quat-free lotions delivered improved moisturization versus the quat-containing benchmark lotion across all Fitzpatrick skin phototype categories.
Conclusion
In conclusion, therapeutic cationic lotions play a crucial role in the treatment of dry skin. The careful selection of ingredients enables the formulation of quat-free lotions that deliver effective moisturization, sensory profiles and other benefits, such as biodegradability and high-percent naturality.
As demonstrated in the present study, lasting moisturization is achievable with the use of cationic emulsifiers such as brassicamidopropyl dimethylamine and BVE. It is therefore suggested that lotions containing these biodegradable amines could address the gap in the market for quat-free cationic therapeutic lotions.
Acknowledgements: The authors wish to thank Mike Fevola, Lynda Johnson and Gia Storti for helpful discussions, and acknowledge the services of Dermico, LLC, for the clinical study.
Footnotes
a Baby Magic is a brand from Naterra International, Inc.
b Corneometer CM 825, Courage + Khazaka
c Ivory is a product of P&G
References
1. Gade, A., Matin, T. and Rubenstein, R. (2022). Xeroderma. PubMed; StatPearls Publishing. Available at https://www.ncbi.nlm.nih.gov/books/NBK565884/
2. Esposito, C. (2021, Oct 6). Iconic Baby Magic brand turns 70. Happi. Available at https://www.happi.com/breaking-news/iconic-baby-magic-brand-turns-70/
3. Curel.com. (2016). Curel Skincare: History. Kao. Available at https://www.curel.com/en-us/about/curel-history/
4. Sakamoto, K., Lochhead, R.Y., Maibach, H.I. and Yamashita, Y. (2017). Lamellar gel networks. In Iwata, T. (ed.), Cosmetic Science and Technology: Theoretical Principles and Applications. Elsevier.
5. Fluhr, J.W., Darlenski, R., Daehnhardt-Pfeiffer, S. and Albrecht, M. (2024). Impact of multilamellar formulations on stratum corneum lipid organization and epidermal lipid barrier enhancement (Part II). Intl J Cos Sci, 46(4), 578–589; https://doi.org/10.1111/ics.12971
6. Cunningham, G.E., Alberini, F., Simmons, M.J. and O’Sullivan, J.J. (2021). Understanding the effects of processing conditions on the formation of lamellar gel networks using a rheological approach. Chem Engineering Sci, 242, 116752. Available at https://doi.org/10.1016/j.ces.2021.116752
7. Carignan, C. (2023, May 13). Disinfectants and cleaning products harboring toxic chemicals are widely used despite lack of screening for potential health hazards. Lake County News. Available at https://tinyurl.com/5n8u74d9
8. Arnold, W.A., et al. (2023). Quaternary ammonium compounds: A chemical class of emerging concern. Environmental Sci & Tech, 57(20); https://pubs.acs.org/doi/10.1021/acs.est.2c08244
9. Dept of Toxic Substances Control. (Accessed 2025, Jun 7). Early-stage SCP projects. Cleaning and personal care products containing quaternary ammonium compounds. Available at http://dtsc.ca.gov/scp/early-stage-scp-projects/#Quaternary_Ammonium_Compounds
10. Gidado, I.M., Qassem, M., Triantis, I.F. and Kyriacou, P.A. (2022). Review of advances in the measurement of skin hydration based on sensing of optical and electrical tissue properties. Sensors, 22(19), 7151; https://doi.org/10.3390/s22197151
11. Fevola, M.J. (2019). The future of cationic surfactants: 100% Bio-based cationic conditioning agents. Premium Beauty News. Available at https://www.premiumbeautynews.com/en/the-future-of-cationic-surfactants,14556
12. Anastas, P.T. and Warner, J.C. (2023, Oct 31). Green chemistry: Theory and practice. Oxford University Press. Available at https://doi.org/10.1093/oso/9780198506980.001.0001
13. Burgo, R. and Parker, J. (2017). Hair conditioning cosmetic compositions containing a mixture of amidoamines. US Pat 9662288. Available at https://patents.google.com/patent/US9662288B2/en
14. Ajayi, O., Davies, A. and Amin, S. (2021). Impact of processing conditions on rheology, tribology and wet lubrication performance of a novel amino lipid hair conditioner. Cosmetics, 8(3), 77; https://doi.org/10.3390/cosmetics8030077
15. Burgo, R. (2012). Non-petrochemically derived cationic emulsifiers that are neutralized amino acid esters and related compositions and methods. US Pat 8105569B2. Available at https://patents.google.com/patent/US8105569B2
16. Nebus, J., Schmalenberg, K. and Wallo, W. (2009) The effectiveness of an oatmeal lotion in improving and maintaining barrier function and moisture levels of moderate to severe xerosis. J Amer Acad Dermatol, 60(3) https://www.jaad.org/article/S0190-9622(08)01848-3/abstract