The fundamental benefits sought by hair care consumers relate to cleansing and care, and with the exception of cleansing, most of these benefits are delivered by conditioning ingredients deposited onto hair to change surface properties such as friction and surface energy. However, simply adding more ingredients to a formula will result in higher costs and may not even improve product performance, especially for a rinse-off product. To develop a hair care product with a high performance-cost profile, knowledge of the deposition efficiency of ingredients is key. In addition, correlating this deposition profile with consumer feedback will provide important guidance for formulators as they develop and optimize hair care products. In relation, traditional techniques to measure deposition are reviewed here, and a novel approach is introduced.
Measuring Deposition
During washing, deposition and rinse-off are opposing behaviors. The driving force behind an ingredient’s deposition is related to its affinity to the hair surface. This is affected by chemical and physical properties such as hydrophobicity, charge type and density, molecular weight, particle size, conjugation/inert action with other ingredients, etc.1 Logically, if the affinity of an ingredient to the hair’s surface is stronger than the forces rinsing it away, the ingredient will remain on the hair surface. Many studies have been conducted to increase this deposition onto hair.2–6 To study deposition efficiency, a reliable, easy-to-use quantitative method is critical and thus far, two approaches generally are used: direct measurement and indirect measurement with extraction.
Direct measurement: Direct measurement analyzes treated hairs for the presence of ingredients. For example, x-ray fluorescence (XRF) can measure silicone deposits on hair by image analysis;7 however, XRF is limited to only silicone ingredients, and it cannot differentiate between pre-existing silicones on hair and those applied by test products. Thus, image analysis methods are usually not quantitative or suitable for routine deposition studies.8
Indirect measurement with extraction: The indirect approach, which is more commonly used, analyzes extracts taken from a treated hair sample. The test product containing the target ingredient is applied to hair via a predetermined protocol, the treated hair is extracted using organic solvent, then the extract is collected and measured to determine the ingredient(s) and amount(s) present. Depending on the polarity of the target ingredient, different solvents can be used to prepare the test samples. For example, to analyze silicone, toluene and methyl isobutyl ketone (MIBK) often are used; for cationic surfactants, trichloromethane or methanol. Thus, to analyze silicone and quats, the preparation of samples must be done separately, which is time-consuming.
Also, unfortunately, since the hair samples are usually real human hair, and each strand has its own history and properties, it is difficult to know the efficacy of the extraction of chemicals from the hair tresses. If the affinity of ingredients to hair is too strong, inefficient extraction can result and lead to variations in the analytical results, misleading development work and ultimately negating the value of performing the analysis in the first place. The data obtained, therefore, is not 100% quantitative, nor does it provide a solid comparison for other hair samples. The information obtained can be useful to explain existing hypotheses, but may not serve as a guide to formulation development. The only way to resolve this issue of reliability is to eliminate the extraction step altogether.
Measuring via Rinse-off
Here, the authors present a new approach that is also indirect but does not require solvent extraction. Simply put, since the original amount of an ingredient applied to hair is known, if the amount present in the rinse water can be determined, the deposited amount can be calculated by subtracting the rinsed portion from the total applied amount. Unlike extraction, accurately analyzing the chemicals in the rinse water is not a technical challenge; and although the variability of human hair is not possible to eliminate, the standard deviations of the final results from triplicate measurements fell within acceptable ranges in the protocol described. Moreover, since the new method employs no solvents, the hair samples were not damaged and could therefore be re-used, to some extent, to conduct different deposition measurements and make the results more comparable.
To test this approach, the authors measured the amount of polydimethylsiloxane (PDMS) silicone deposited onto hair. Qualitative 1H nuclear magnetic resonance (NMR) was used for ingredient analysis, as it is quick and highly sensitive with a relatively easy sample preparation. Silicone was chosen because it is the most commonly used ingredient to reduce hair surface friction for conditioning benefits. It also can be analyzed by 1H NMR without requiring further chemical treatments, such as digestion or chemical derivation. Further, it is easier to distinguish the proton signal of silicone, which appears at around 0 ppm, and at which point there is also almost no overlap with other signals.
Materials and Methods
Hair samples: Hair tresses collected from Asian women and having no chemical damage were purchased for this study; they measured approximately 20 cm in length, 5.5 cm in width and 16.0 g in weight. The samplesa were washed with a cleansing shampoo before use and measured with a combing testerb to determine their required combing force (RCF) values. This confirmed the tresses were clean and that their RCF values before treatment did not vary widely. Note that hereinafter, the RCF value before use will be referred to as the wet baseline.
Researchers used 24 hair tresses per test divided into eight groups of three tresses each for statistical analysis. The cleanness and homogeneity criteria were: an average wet baseline RCF value for all 24 samples within a +10% difference vs. the previous test’s wet baseline RCF value; and the relative standard deviation (RSD) for all 24 tresses and for three samples in one group within 10%.
Combing tests: The combing testerb utilized was equipped with: two shower heads; two combing arms to which brushes were connected vertically to a potential displacement meter—hereinafter referred to as the arms; and a hair tress holder connected to a load cell (see Figure 1). As the mode of action, the two combing arms driven by compressed air strike horizontally to hold a hair tress between brushes, then strike vertically by a high-precision servomotor to comb through the hair tress. The vertical displacement of the arms and the load on the hair tress holder during combing, i.e. RCF, were recorded every 10 milliseconds.
Preparation phase: The measurement process consisted of two phases: preparation and treatment. The procedure for preparation was: 1. Set a hair tress on the holder; 2. Apply a warm water shower (40 + 5°C) at a flow rate of 70 + 1 mL/sec onto the tress from both shower heads for 15 sec; and 3. Comb the tress with the arms five times while applying the warm water shower. After completion of this preparation phase, a plastic tray (350 x 290 x 120 mm) was placed into the combing tester to collect rinse water for quantifying the target ingredients in a later stage of the experiment.
Treatment phase: The procedure for the treatment phase was: 1. Apply 1.5 mL of the test product on both sides of the tress with a syringe; 2. Spread the test sample from the root to the tip of the tress by clamping the tress between one’s gloved palms; 3. Comb the tress with the arms five times without rinsing it; 4. Comb the tress with the arms 10 times while applying the warm water rinse; and 5. Comb the tress with the arms five times without rinsing. After completing this treatment phase, the plastic tray with rinse water was removed from the combing tester.
The tress was then removed from the holder, hung on a rack, dried for 24 hr and measured for its dry combing force value the next day by combing the tress with the arms five times without rinsing. As noted, three hair tresses were used for each test, and the brushes were removed from the arms and washed each time after completing measurements for three tresses to avoid cross-contamination. The disposable gloves also were washed in the rinse water collected in the plastic tray after each treatment to minimize residue, and replaced with new ones every three tresses—again, to minimize cross-contamination.
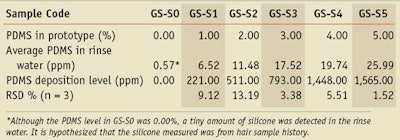
Test shampoos: Six shampoo samples were prepared for this study. The basic ingredients used were sodium lauryl ether sulfate (16.0%), glycol distearate (2.0%), polyquaternium-10 (0.5%), guar hydroxyproyltrimonium chloride (0.2%) and others such as preservatives and perfume. The single variable in these formulae was the dimethicone level; the shampoo samples were prepared with 0.0 (GS-S0), 1.0 (GS-S1), 2.0 (GS-S2), 3.0 (GS-S3), 4.0 (GS-S4) and 5.0% (GS-S5) dimethicone.
Chemicals: The chemicals purchasedc for NMR analysis included: p-dinitrobenzene, 98%+ GC grade; dimethyl sulfoxide–d6, 99.9%; and chloroform-d, 99.8%.
Sample Preparation, 1H NMR Conditions and Calculations
All rinse-off water was collected and accurately weighed following the protocol described in the combing test. At this point, the total obtained per tress was about 2 L. Considering the sensitivity of quantitative proton NMR analysis; however, a concentration step also was required, for which about 50 g of rinse water was accurately weighed out from five different sampling sites (50 g = 10 g x 5) after recovered rinse water was mixed evenly (unless otherwise specified).
The collected water was evaporated in a water bath, and the residue was dissolved in approx. 5 mL CDCl3. Then, 20 mg of p-dinitrobenzene solution (approx. 1.8% in DMSO-d6) was added as an internal standard, mixed well, and a final proper amount (approx. 1.5 mL) of solution was transferred into an NMR tube for quantitative analysis.
Quantitative proton NMR was carried out using a 500-MHz spectrometerd with a normal proton pulse sequence that was modified by extending the delay time to about 20 sec. A typical 1H NMR spectrum is shown in Figure 2. The proton signal of silicone is around 0 ppm, and the internal standard p-dinitrobenzene is around 8.4 ppm. The values of these two signals were used for calculations (see Eq. 1 and Eq. 2).
To calculate the amount of silicone collected in the rinse water from the NMR analysis, or Wsam (mg), Eq. 1 was used:
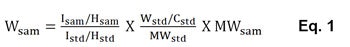
where Isam = integrated value of silicone proton signal at 0 ppm; Istd = integrated value of internal standard proton signal at 8.4 ppm; Hsam = proton number of one dimethicone unit; Hstd = proton number of p-dinitrobenzene; Wstd = p-dinitrobenzene amount used in the test sample for NMR analysis (mg); Cstd = concentration of p-dinitrobenzene; MWstd = molecular weight of p-dinitrobenzene; and MWsam = molecular weight of one dimethicone unit. To calculate the silicone deposition amount on hair (D, in ppm), Eq. 2 was used:
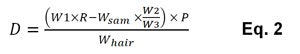
where W1 = sample amount used in the combing test (mg); R = weight ratio of silicone in product (%); Wsam is the result from Eq. 1; W2 = total rinse water (mg); W3 = amount of rinse water used for proton NMR sample preparation (mg); P = 0.8, i.e., ratio of used hair surface in total hair tress; and Whair = weight of hair tress (kg).
Results: Method Validation
As noted previously, the described method is an indirect approach to analyze the amount of an ingredient collected in rinse water, which is then subtracted from a known amount applied to determine final deposition results. Thus, based on the authors’ previous experience with silicone deposition, a validation plan for this method was designed as follows.
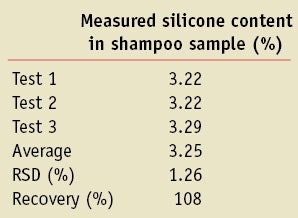
A shampoo containing 3.0% silicone was developed, and 1.5 g of the product sample was dissolved in 2 L of water to mimic the concentrations of silicone found in the rinse-off water from the deposition tests. Again, considering the sensitivity of quantitative 1H NMR, approx. 50 g of the rinse-off water was accurately weighed, the water was evaporated following the sample preparation procedure described in the experimental part, and samples were prepared for proton NMR measurements. These measurements were conducted in triplicate to check recovery and reproducibility. As shown in Table 1, recovery of the silicone polymer was 108% and the RSD% was 1.26%.
Results: Silicone Deposition
The six shampoo samples containing 0.0, 1.0, 2.0, 3.0, 4.0 and 5.0% PDMS were prepared, and each sample was applied on at least three different hair tresses as described in the experimental part. The rinse water was analyzed with proton NMR following the sample preparation procedure described; the silicone deposition results are summarized in Table 2.
Comparing the silicone deposition amounts of samples with different PDMS levels, i.e., 0.0–5.0%, it is obvious that initially, the amount of silicone deposited increased with increasing silicone content (see Figure 3). However, at the highest silicone levels, the trend for increased deposition leveled off and finally reached saturation. Specifically, when the silicone level in shampoo was increased from 1.0 to 3.0%, its deposition increased by about 280 ppm. When silicone levels increased from 3.0 to 4.0%, deposition increased by almost 650 ppm; however, when silicone levels increased from 4.0 to 5.0%, deposition increased only by about 110 ppm.
Results: Dry Hair Combing Force
It is well known that silicone is highly effective at reducing hair surface friction, making it easier to comb. Correlating deposition data with combing force data thus provides further evidence of the usefulness of the silicone deposition test. Figure 4 compares the dry hair combing forces of samples treated with GS-S0 and GS-S1, revealing that silicone deposited onto hair reduced the dry hair combing force significantly.
Overall, with increasing silicone deposition, dry hair combing forces decreased; the combing force of GS-S3 showed significant reduction versus GS-S1 and GS-S2, at a 95% confidence level. However, despite of significant silicone deposition increase from GS-S3 to GS-S4, the dry hair combing force reduced but not significantly. To explain this phenomenon, it is hypothesized that once hair’s surface has been occupied by silicone and other ingredients, the excess silicone deposited on top may not significantly contribute to friction reduction. This is also consistent with sensory feedback that too high silicone levels in a product do not help hair’s dry feel.
Conclusion
Data obtained from quantitative proton NMR analysis, along with its correlation with measured dry hair combing force results, indicates the new rinse-off method described is highly effective for hair care ingredient deposition analysis. Although only silicone deposition results were disclosed and discussed in this paper, the ingredients that can be analyzed using this approach are unlimited. The use of analytical instruments is also not limited to proton NMR.
Furthermore, this approach opens the door to monitor the rinse-off behavior of products and ingredients dynamically. In conclusion, this novel approach can serve as a more accurate, safe and environmentally friendly, non-destructive tool for guiding rinse-off hair product development.
References
1. MA Brown, TA Hutchins, CJ Gamsky, MS Wagner, SH Page and JM Marsh, Liquid crystal colloidal structures for increased silicone deposition efficiency on color-treated hair, Intl J Cos Sci 193–203 (32) (2010)
2. S Nanavati and A Hami, A preliminary investigation of the interaction of a quat with silicones and its conditioning benefits on hair, J Soc Cosmet Chem 135–148 (45) (1994)
3. Z Hu, M Liao, Y Chen, Y Cai, L Meng, Y Liu, N Lv, Z Liu and W Yuan, A novel preparation method for silicone oil nanoemulsions and its application for coating hair with silicone, Intl J Nanomedicine 5719–5724 (7) (2012)
4. CL Torre, B Bhushan, JZ Yang and PM Torgerson, Nanotribological effects of silicone type silicone deposition level and surfactant type on human hair using atomic force microscopy, J Cosmet Sci 37–56 (57) (2006)
5. JV Gruber, BR Lamoureux, N Joshi and L Moral, Influence of cationic polysaccharides on polydimethylsiloxane (PDMS) deposition onto keratin surfaces from a surfactant emulsified system, Colloids and Surfaces B: Biointerfaces 127–135 (19) (2000)
6. H Nazir, L Wang, G Lian, S Zhu, Y Zhang, Y Liu, G Ma, Multilayered silicone oil droplets of narrow size distribution: Preparation and improved deposition on hair, Colloids and Surfaces B: Biointerfaces 42–49 (100) (2012)
7. AD Smedt, IV Reeth, S Marchioretto, DA Glover and J Naud, Measurement of silicone deposited on hair, Cosm & Toil 112 (39) (1997)
8. V Gruber, BR Lamoureux, N Joshi and L Moral, The use of X-ray fluorescent spectroscopy to study the influence of cationic polymers on silicone oil deposition from shampoo, J Cosmet Sci, 131–136 (52) (2001)