Editor's note: What can differences in structure and function within a chemical family reveal? And how might these insights prove useful? In this installment of "Comparatively Speaking," Anthony J. O'Lenick, Jr., illustrates with a closer look at homologous and analogous polymers. For more in this comparative series, see Comparatively Speaking.
The structure/function relationship between chemicals is one very basic reason to study a series of related chemicals, and to use their differences to determine trends within a chemical family. This is an important undertaking in organic chemistry, but crucial to polymer chemistry.
Two types of relationships prove most fruitful for studying effects and making related molecules. One is the investigation of a homologous series of compounds; the other is an analogous series of related compounds.
Homologues
A homologue is a compound belonging to a series of compounds that differs from one other by a repeating unit such as a −CH2− group. This means that in the series, there is no substitution of one functional group for another, nor is there any elimination of a functional group.
An example in organic chemistry is hydrocarbons. Within one homologous series, one can define butane, heptane hexane, and so on. One can look at their boiling points, freezing points and perhaps even solubility in various solvents.
This type of study can be applied to polymers. Consider the following cetyl dimethicone molecule (see Figure 1):
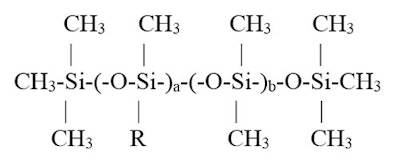
where R is -(CH2)15-CH3
This molecule is an alkyl dimethicone. The R group is oleophillic, contained in the a group. The silicone-soluble group includes everything within the b group and the terminal ends. As a increases relative to b, the % of alkyl group likewise increases. Consequently, if one considers solubility in differing polarity oils, one can relate % alkyl to solubility.
It is worth noting that a series of homologs can also be created in which the C16 alkyl is altered with other alkyl chains (C4-C32). These materials would not only exhibit an altered solubility, but an altered melting point. Put another way, homologues are materials in which the number of groups are altered, but no new groups are added and no existing groups are removed from the molecule. In contrast, analogues (described later) are materials in which one or more groups are replaced by another group.
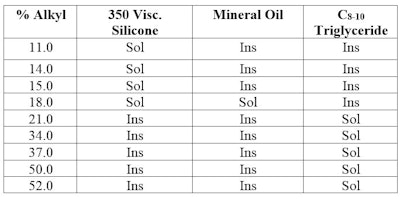
Table 1 shows the results of such an experiment. Not only does it show there are cetyl dimethicone polymers that are soluble in 350-viscosity silicone fluid, but one that is soluble in both mineral oil and silicone; and those that are soluble in C8-10 triglyceride. And this is despite the fact that the three solvents are not soluble in each other. A graphic representation is shown in Figure 2.
A prediction was made nearly 20 years ago for an alkyl dimethicone that is soluble in both silicone and mineral oil; this was described in an article outlining a three-dimensional HLB system.1 Such compatibility is fundamental to good formulating.
For example, the selection of the proper alkyl silicone to minimize syneresis in pigmented products, stabilize an emulsion oil phase, or modify aesthetics is enhanced if the particular alkyl silicone is soluble in the phase in which it will be formulated.
Different alkyl dimethicone polymers can be evaluated by adding them to formulated oil phases having several components, to ultimately arrive at the best candidate for the mixed system.
Analogues
In contrast to homologues, analogues are compounds belonging to a series of compounds that differ from one other by replacing one functional group with another functional group. Examples from organic chemistry are the analogous surfactants sodium laureth-2 sulfate and sodium laureth-2 phosphate.
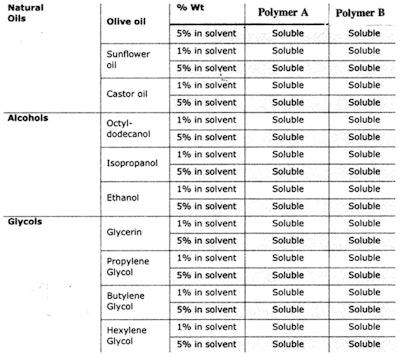
This type of study can be applied to polymers, too. Consider the same cetyl dimethicone molecule, shown in Figure 1; but this time, a and b are kept constant and R is changed as follows.
For Polymer A: R is –(CH2)3-O-(CH2CH2O)8H
For Polymer B: R is –(CH2)3-O-CH2CH-(OH)-CH2-N+-(CH3)3 Cl-
Initially, one might expect significant differences in solubility since the quat, not the PEG, is charged. However, the solubility of these two polymers in several solvents is very similar, as shown in Table 2.
Take-home Message
The bottom line? When working with closely related chemicals, do not lose sight of looking for similarities and differences of analogues and homologues.