Hyperpigmentation is a disorder characterized by an overproduction of melanin in the skin. Common hyperpigmentation disorders include, but are not limited to, post-inflammatory hyperpigmentation, melasma and solar lentigines.1
Post-inflammatory hyperpigmentation typically results from inflammation, e.g. it is caused by acne or skin injury, and it occurs most frequently with patients of Fitzpatrick skin types IV-VI.1 Melasma is characterized by patches of discoloration—typically facial—as a result of pregnancy-related hormonal changes, oral contraceptives or sun exposure.2
Pathak et. al., hypothesizing that sun exposure exacerbates hyperpigmentation, showed ultraviolet (UV) irradiation appeared to cause cellular injury, leading to an increased synthesis of proteins, melanosomes and tyrosinase that ultimately yielded new melanin.3
Due to sunlight’s influence in hyperpigmentation, sunscreen is a common part of treatment in clinical trials. Sunscreen is used for photoprotection not only for hyperpigmentation, but for preventing sunburn, photocarcinogenesis, photoimmunosuppression and photoaging.4
Two common forms of sunscreen, chemical and physical, mechanistically protect the skin in similar ways. In chemical sunscreens, chemicals such as avobenzene or oxybenzene absorb UV light of certain energies. Physical sunscreens use zinc oxide and titanium dioxide, which also absorb electrons of UV light within their electronic structure.4
Sunscreen Protection Factor (SPF) provides a quantitative value on the effectiveness of a sunscreen formulation. By definition, it is the minimal erythema dose in sunscreen-protected skin. The SPF indicates UVB protection—agents in sunscreen provide maximum absorption in the UVB spectrum.4
In 1983, Vasquez et. al. showed in a double-blinded trial of sunscreen for melasma treatment that 96% of patients showed improvement in hyperpigmentation when using sunscreen, in contrast to 81% of patients using a placebo. The trial established sunscreen’s potentially beneficial role in treating hyperpigmentation.5
Numerous clinical trials utilize sunscreen as a part of treatment when investigating hydroquinone, azelaic acid and niacinamide’s effects as depigmenting agents. Our objective was to understand the role sunscreen plays in the treatment of hyperpigmentation through revisiting clinical trials.
Methods
The primary topical depigmenting agents studied were hydroquinone, azelaic acid, ascorbic acid, niacinamide and retinol and its derivatives. For published studies, we conducted a systematic literature search on Medline, using search strings of depigmenting agents, i.e. ‘hydroquinone’ and the term ‘clinical trial.’ Included clinical studies were limited to January 2009 to the present (primo July 2014), written in English, which had 10 or more human participants.
For published trials, we narrowed down to those that were comparative, i.e. sunscreen versus depigmenting agent or sunscreen versus placebo, to understand sunscreen’s role in hyperpigmentation treatment.
We examine study design, objective, intervention, how investigators use sunscreen, measurements of efficacy and results.
Results
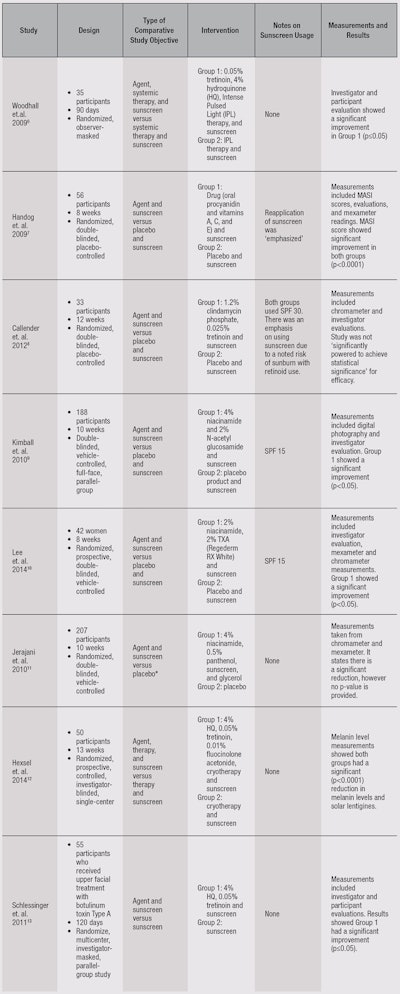
Table 1 provides an overview of methods for published comparative clinical trials using depigmenting agents against therapy, placebo and/or sunscreen. Forty studies were examined in total: 22 for hydroquinone, five for ascorbic acid, one for azelaic acid, five for niacinamide and seven for retinol. Thirty-one of the 40 trials used sunscreen as part of the treatment: 20 for hydroquinone, three for ascorbic acid, zero for azelaic acid, three for niacinamide, and five for retinol. Eight of the 40 studies used sunscreen comparatively: Three for hydroquinone, one for ascorbic acid, zero for azelaic acid, three for niacinamide, and one for retinol.
For comparative trials, number of patients ranged from 33 to 207. Duration of trials ranged from eight to 16 weeks; and five of eight trials were double-blinded. For studies providing information on sunscreen, SPF ranged from 15 to 30; two trials emphasized the reapplication of sunscreen. Measurements included investigator and patient evaluations, MASI scores, mexameter and chromameter measurements and digital photography. Six trials reported statistically significant results.
The comparative trials we looked into tested an agent and sunscreen against either a placebo and/or sunscreen. Of eight comparative trials using sunscreen, four were conducted by using agent and sunscreen versus placebo and sunscreen, two used agent, therapy and sunscreen versus placebo, therapy and sunscreen, one used agent and sunscreen versus a control lotion (sunscreen use in control group was not specified), and one used agent and sunscreen versus sunscreen.
Discussion
Since most studies for hyperpigmentation utilize sunscreen, we wanted to understand its efficacy for treating hyperpigmentary disorders.
To understand sunscreen’s independent role in treatment, we examined the comparative studies, which in some form separated sunscreen from the depigmenting agent either through using it in placebo treatment or agent on its own.
Vasquez et. al. in 1983 performed a study establishing a relationship between sunscreen as a beneficial addition or treatment to hyperpigmentation.5 This trial, however, took place more than 20 years ago. While it did show that sunscreen improved hyperpigmentation, it lacked statistical significance testing. Therefore, we looked toward current trials to understand sunscreen’s impact on treating hyperpigmentation.
Because of the comparative study setup—agent and sunscreen versus placebo and/or sunscreen—in these trials, it’s difficult to come to a conclusion on the efficacy of sunscreen, placebo, or depigmenting agent (and in some cases, the systemic therapy).
Based on our results, we cannot say with
certainty how effective sunscreen is in the treatment of hyperpigmentation.
Ideally, studies that perform a double-blinded, randomized, comparative study of depigmenting agent versus placebo versus sunscreen would help clinicians understand efficacy independently. From there, seeing if using sunscreen in conjunction with depigmenting agents improves efficacy is another step. Studies addressing sunscreen’s efficacy in treating hyperpigmentation are welcomed.
References
- Baumann, L., Rodriguez, D., Taylor, S. C., & Wu, J. (2006). Natural considerations for skin of color. Cutis, 78(6 Suppl), 2–19. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17354519
- Cestari, T., Arellano, I., Hexsel, D., & Ortonne, J. P. (2009). Melasma in Latin America: options for therapy and treatment algorithm. Journal of the European Academy of Dermatology and Venereology : JEADV, 23(7), 760–72. doi:10.1111/j.1468-3083.2009.03251.x
- PATHAK, M. A., RILEY, F. C., & FITZPATRICK, T. B. (1962). Melanogenesis in human skin following exposure to long-wave ultraviolet and visible light. The Journal of Investigative Dermatology, 39, 435–43. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/13941837
- Wolf, R., Tuzun, B., & Tuzun, Y. (2001). Sunscreens. Dermatologic Therapy, 14(3), 208–214. doi:10.1046/j.1529-8019.2001.01027.x
- Vázquez, M., & Sánchez, J. L. (1983). The efficacy of a broad-spectrum sunscreen in the treatment of melasma. Cutis, 32(1), 92, 95–6. Retrieved from http://europepmc.org/abstract/MED/6349944
- Woodhall, K. E., Goldman, M. P., Gold, M. H., & Biron, J. (2009). Benefits of using a hydroquinone/tretinoin skin care system in patients undergoing intense pulsed light therapy for photorejuvenation: a placebo-controlled study. Journal of Drugs in Dermatology : JDD, 8(9), 862–7. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19746679
- Handog, E. B., Galang, D. A. V. F., de Leon-Godinez, M. A., & Chan, G. P. (2009). A randomized, double-blind, placebo-controlled trial of oral procyanidin with vitamins A, C, E for melasma among Filipino women. International Journal of Dermatology, 48(8), 896–901. doi:10.1111/j.1365-4632.2009.04130.x
- Callender, V. D., Young, C. M., Kindred, C., & Taylor, S. C. (2012). Efficacy and Safety of Clindamycin Phosphate 1.2% and Tretinoin 0.025% Gel for the Treatment of Acne and Acne-induced Post-inflammatory Hyperpigmentation in Patients with Skin of Color. The Journal of Clinical and Aesthetic Dermatology, 5(7), 25–32. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3396458&tool=pmcentrez&rendertype=abstract
- Kimball, A. B., Kaczvinsky, J. R., Li, J., Robinson, L. R., Matts, P. J., Berge, C. A., … Bissett, D. L. (2010). Reduction in the appearance of facial hyperpigmentation after use of moisturizers with a combination of topical niacinamide and N-acetyl glucosamine: results of a randomized, double-blind, vehicle-controlled trial. The British Journal of Dermatology, 162(2), 435–41. doi:10.1111/j.1365-2133.2009.09477.x
- Lee, D. H., Oh, I. Y., Koo, K. T., Suk, J. M., Jung, S. W., Park, J. O., … Choi, Y. M. (2014). Reduction in facial hyperpigmentation after treatment with a combination of topical niacinamide and tranexamic acid: a randomized, double-blind, vehicle-controlled trial. Skin Research and Technology : Official Journal of International Society for Bioengineering and the Skin (ISBS) [and] International Society for Digital Imaging of Skin (ISDIS) [and] International Society for Skin Imaging (ISSI), 20(2), 208–12. doi:10.1111/srt.12107
- Jerajani, H. R., Mizoguchi, H., Li, J., Whittenbarger, D. J., & Marmor, M. J. (n.d.). The effects of a daily facial lotion containing vitamins B3 and E and provitamin B5 on the facial skin of Indian women: a randomized, double-blind trial. Indian Journal of Dermatology, Venereology and Leprology, 76(1), 20–6. doi:10.4103/0378-6323.58674
- Hexsel, D., Hexsel, C., Porto, M. D., & Siega, C. (2014). Triple combination as adjuvant to cryotherapy in the treatment of solar lentigines: investigator-blinded, randomized clinical trial. Journal of the European Academy of Dermatology and Venereology : JEADV. doi:10.1111/jdv.12484
- Schlessinger, J., Kenkel, J., & Werschler, P. (2011). Further enhancement of facial appearance with a hydroquinone skin care system plus tretinoin in patients previously treated with botulinum toxin Type A. Aesthetic Surgery Journal / the American Society for Aesthetic Plastic Surgery, 31(5), 529–39. doi:10.1177/1090820X11411579