Hair treatment and care traditionally involves two distinct steps. First, cleansing with primarily anionic or amphoteric surfactants, followed by rinsing. Then, applying a conditioning formula that contains a cationic surfactant and generally cetyl and stearyl alcohol. Attempts to combine these steps into a single use product is not an easy proposition.
The challenge is that the shampoo has an anionic charged surfactant and is hydrophilic, and the conditioner is cationic and contains structured hydrophobic material. However, various attempts have been made to overcome the incompatibilities of these materials to integrate shampoos and conditioners in so-called 2-in-1 products.
One approach is to select "soft anionics" and "soft cationics" to formulate mixtures that are stable. Another approach is to “hide” the conditioner in an emulsion that breaks to deliver the conditioner when the mixture is rinsed off. The former is complexation and the latter is coacervation—these are the focus of this installment of "Comparatively Speaking."
Complexation
The interaction of anionic/cationic materials is critical to formulations. Most formulators of 2-in-1 shampoos understand that the indiscriminate mixing of anionic and cationic materials can result in undesired, insoluble “gunky” solids. Anionic and cationic materials that are incompatible when mixed together are considered "hard complexes." As the expression implies, these cationic and anionic compounds possess properties that, when added together, form insoluble complexes such as salts.
Good foaming, viscosity-building and cleansing results also have been achieved with complexe such as cocamidoproyl trimethyl ammonium chloride.
In contrast, anionic and cationic compounds that can be mixed over a wide range of ratios and provide a clear, viscous, high-foaming complex are defined as "soft complexes." Optimized soft complexes have many desirable properties including high levels of foam, viscosity-building without alkanolamides, conditioning properties, and low levels of eye and skin irritation.1
Quaternium compounds can be classified as hard or soft based on their ability to form gels with anionic systems. In cationic systems, those that form a gel at near stoichiometric amounts are classified as soft quats; those that form precipitates of haze without appreciable viscosity build-up are classified as hard quats. Soft quats can produce foam in the systems they gel, albeit at levels below the volume of foam generated by the anionic, per se.
Good foaming, viscosity-building and cleansing results also have been achieved with some complexes. Specifically, cocamidoproyl trimethyl ammonium chloride has been found to be very effective.
Coacervation
By forming a coacervate, the oil added to a formulation neither separates out nor defoams the shampoo, providing a great approach to making 2-in-1 shampoos. Coacervates are organic-rich droplets formed via liquid-liquid phase separation.
Coacervation is a phenomenon that produces coacervate colloidal droplets. When coacervation happens, two liquid phases will co-exist: a dense, polymer-rich phase (coacervate phase or coacervate droplets) and a dilute, polymer-deficient phase (dilute phase). Coacervate droplets can measure from 1 to 100 micrometers across, while their soluble precursors are typically on the order of less than 200 nm. The name coacervate derives from its Latin origin, meaning "to assemble or cluster."2, 3
See related: Deposition from Conditioning Shampoo, Optimizing Coacervate Formation
Silicones are used as conditioning agents in shampoos, where they have been found to deposit at high rates onto the surface of the hair, especially if combined in the product with a cationic (positively-charged) polymer (referred to on labels as polyquaterniums). This mechanism of conditioning is known as dilution deposition or the “Lochhead Effect.”4 Due to this property, they have played a major role in the innovation of 2-in-1 shampoos, and are still used in these formulations today.
Prof. Robert Lochhead, Ph.D., a pioneer in this arena, provides key insights about how this dynamic works in understandable terms: “In a 2-in-1 product, the catatonic surfactants remain suspended in all the suds while the shampoo is working to break down oils and dirt. Then, when you rinse the shampoo out, the surfactants are ‘triggered’ by the water to bind to the hair, while only the dirt and oil washes away.”5
Common ingredients to make this process effective are softening silicones like dimethicone and polymers like polyquaternium-10. It is believed that the hair-bodying resin precipitates upon dilution of the shampoo composition and application to the hair, whereupon the resin coacervates with the siloxane and the coacervate deposits on the hair strands.5
The consequence of incorporating the silicone oil as a preformed aqueous emulsion is that its particle size is small. This silicone is insoluble and remains emulsified in the overall composition. Incorporating the silicone oil in this way makes the manufacture of the compositions easier. It also reduces the antifoam action of the silicone oil and leads to compositions of greater stability.6 As the shampoo is rinsed off, the aqueous emulsion breaks, releasing the conditioning oil.
By forming a coacervate, the oil added to a formulation neither separates out nor defoams the shampoo, providing a great approach to making 2-in-1 shampoos.
Lochhead provides additional insights into coacervate selection by computer modeling. Most conditioning shampoos depend upon the deposition of a polymer-surfactant coacervate to confer good wet-combing and manageability. Complex coacervate formation is crucially dependent on the molecular characteristics of the polymer and surfactant species, and is significantly affected by the presence of other ingredients such as co-surfactants and dissolved salts.
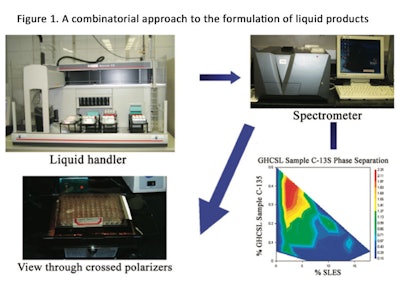
The optimization of these systems presents a challenge to the formulator due to the astronomical number of possible compositions with different performance outcomes.7 Figure 1 shows a suggested approach to formulating liquid products using a combinatorial approach. Table 1 also provides the results of a literature search that relates to coacervate technology.
Clearly there are many patents over the years, which continue to the present time.