Marketers become excited when presented with ingredients promising benefit claims to surpass that of their competition. However, the same ingredients are sometimes employed by competitors-and consumers seek formulations that deliver desirable and perceivably differentiated benefits. The formulator is thus presented with the challenge of achieving better results when their star ingredient is also used by the competition. Opportunities lie in the delivery of those ingredients.
A typical skin care formula may contain from a few to a few dozen ingredients, and some of these ingredients react during manufacturing to form new ones. When the final product is applied, a race ensues among all ingredients to penetrate the skin; the greater the number, the more competitive the race. The star ingredients of the formula also face the same dilemma—competing against all others for skin penetration. So in a nutshell, just because a performance ingredient is in a formula does not guarantee it will penetrate the skin in any quantifiable amount to deliver the claimed benefits. The present article reviews routes to deliver these ingredients to their targets in skin, to improve their chances of penetration for designed activities and final end benefits.
Skin Barrier and Topical Delivery
To understand mechanisms for penetration, first consider some biology. Protection is the skin’s main function, which means stopping any undesirable objects such as bacteria, fungi and chemicals from entering the body. This barrier function is specific to the superficial part of the skin, the stratum corneum (SC), which has recently been shown to include three sharply demarcated layers with distinct barrier properties (see Figure 1).1 The SC consists of cornified keratinocyte (corneocytes) layers attached to each other by corneodesmosomes, with intercellular spaces sealed by lipids. The intracellular space of corneocytes is filled with keratin filaments, filaggrin and their degradation products.
Each corneocyte is encased in a cornified envelope–an insoluble amalgam of proteins highly cross-linked by transglutaminases, the surface of which is tightly bound to intercellular lipids to provide a barrier against the passage of water and water-soluble substances. The SC thus functions as a barrier against foreign insults, as well as a barrier to keep skin hydrated.2 However, topical delivery systems can facilitate the penetration of desirable ingredients.
Topical delivery systems are prevalent in the pharmaceutical industry, yet relatively unreckoned in cosmetics. A topical delivery system or vehicle is defined as the substance that carries a specific active agent into and through the skin. In a broader sense, topical delivery is a strategy to improve the aesthetics and performance of an ingredient or its formulation. The major challenge of topical delivery is the transport of active agents across the skin barrier.3 However, this is not a straightforward path. Depending on the delivery system, the penetration of the active can be variable due to the physicochemical properties of the constituent components of that vehicle.
The selection of the appropriate topical delivery system will depend on the active agent, anatomical site of application, and consumer preferences for aesthetics and skin feel. One cannot simply add a topical delivery system into an existing formulation and expect it to penetrate. In the selection of a suitable delivery system, all ingredients, including those in the base, must be selected with care for their chemical compatibility, stability and competition kinetics during skin penetration from the formulation.
Formula Constituents
Topical formulations typically have three main constituents: the active agent, which provides the claim or story; base ingredients including emulsifiers, thickeners, emollients, moisturizers, stabilizers, preservatives, color, fragrance, etc.; and the medium, i.e., water, anhydrous system, hydroxylic solvents, silicones, etc. All of these influence the kinetics of skin penetration.
The skin penetration of salicylic acid offers an example. Salicylic acid is practically insoluble in water at ambient temperatures. It has better solubility in alcohol and other hydroxylic formulation media such as glycols. In a typical skin care lotion or cream that contains ~80% water, the addition of salicylic acid at 2% for example, even during the manufacturing of the formula at elevated temperatures, may not provide expected levels of efficacy since some or all of it may have crystallized out as fine, imperceptible particles suspended in the matrix of the formulation upon cooling. Such solidified microcrystals would have poor skin penetration. Alternatively, salicylic acid may first be dissolved in alcohol and the resulting solution added to the remaining batch; but again, salicylic acid may separate out as suspended microcrystals as soon as the alcohol dissipates into the water of the base formulation. Clearly, a proper delivery system to prevent such crystallization could provide a solution.
Selecting the correct delivery medium is also of paramount importance. In the case of salicylic acid, it may be dissolved in alcohol or a glycol, i.e., PEG-3 or PEG-4, and the resulting solution may be converted into a gel with a suitable thickening agent. When these are applied topically, two different results may be observed. In the case of alcohol, salicylic acid may appear as a white film on the skin due to the rapid evaporation of alcohol. In the case of a glycol, the formulation would penetrate the skin, leaving no visible residue of salicylic acid. It is worthy to note that chemical analyses of the above formulations would indicate nearly the same amount of salicylic acid content, albeit with a different level of skin penetration or bioavailability kinetics, consistent with their consumer-perceived efficacy variance. As a rule, an ingredient in a liquid or solution form would have a faster rate of penetration than the same ingredient in a solid or a crystalline state.
Most organic compounds used in topical formulations fall broadly into two classifications, lipophilic or hydrophilic, the incompatibilities of which are bridged by surfactant amphiphilic agents. In terms of their topical delivery, a judicious pursuance of pharmaceutical topical delivery technologies in cosmetics, especially skin care, can provide powerful benefits to the consumer.
Generalized Routes
Cosmetic ingredients can be formulated for topical delivery through the SC via many generalized routes (see Figure 2). Regarding safety, note that toxicity is related more to the ingredients, and not necessarily to the depth of skin penetration. This review provides a summary of modern innovations within the past five years. Additional information is available elsewhere (see Additional Reading sidebar).4
Volatile solvents: Volatile components such as alcohols (i.e., ethanol and isopropanol), acetone and silicones in simple formulations including gels or sprays are popular from a cosmetic perspective. However, complex formulation effects may result from the use of volatile excipients in topical formulations.5 A comparative study was conducted of the skin penetration of methylparaben, as a model compound, in combination with diethylene glycol monoethyl ether, dimethyl isosorbide and isopropyl myristate in an ethanol solution. In vitro diffusion experiments using silicone membranes and human epidermis concluded that rapid evaporation of ethanol takes place from the formulations applied at the surface of skin, leaving a saturated residue of the drug in the vehicle. The presence of ethanol clearly influenced the efficiency of the formulation to deliver the material, underlining the application of volatile components to optimize dermal delivery.
Results also showed that the presence of ethanol had little effect on the skin flux of methylparaben, compared with corresponding saturated solutions. The permeation of methylparaben from the saturated ethanol solution in both silicone and skin models showed an initial period of relatively fast permeation, followed by a marked decrease in the permeation rate. This reflected significant ethanol depletion from the formulation, chiefly by evaporation, causing most of the methylparaben to crystallize as a deposited film at the skin surface and thus decreasing its availability to permeate. Studies of the kinetics of ethanol evaporation from the formulations also confirmed these findings.5
Different amounts of the volatile vehicle, such as ethanol, also can influence the skin penetration profile of an active agent. Etofenamate, a non-steroid anti-inflammatory drug used for its analgesic, anti-rheumatic, antipyretic and anti-inflammatory properties, was evaluated for topical administration in a hydroalcoholic gel (5.0% etofenamate). In vitro release and permeation studies revealed that differing the amounts of ethanol influenced the release profiles of etofenamate.6
However, caution must be taken with topical formulas based on volatile vehicles that also act as solubilizing mediums for certain ingredients, especially those that have limited solubility or are insoluble in water. Decreased solubility and higher ambient temperatures increase the rate of evaporation of volatile components from the formulation, in turn varying the skin penetration of such ingredients, along with their deposition or crystallization on the skin. Further, when emollients or moisturizers are present in the formula, they can mask the physical appearance of the film deposited on the skin, which can mislead conclusions regarding actual versus claimed consumer benefits.
Non-volatile silicones: Novel silicone materials also have been investigated for their topical delivery efficacy. An anhydrous semisolid formulation comprising a novel cross-linked silicone polymer in isododecane solvent containing 5% ibuprofen (IBP) was evaluated in vitro for the permeability of IBP in human cadaver skin. Similar formulations prepared with petrolatum, an acrylic or a cellulose polymer, and IBP were compared to a formulation containing a commercial silicone and IBP. The silicone formulation delivered IBP more efficiently than all other formulations in terms of flux, cumulative amount and percent of drug released. An in vivo study conducted on rats also was performed with these formulas and the results obtained demonstrated efficient topical delivery of IBP by the silicone formulation.7
Liquid crystals: Silicone-based liquid crystals containing polyether functional siloxane as a surfactant, silicon glycol copolymer as an oil phase, and water have been studied for their ability to topically deliver retinyl palmitate. Results showed that silicone liquid crystals improved the safety profile and maintained a higher antioxidant activity of retinyl palmitate, thus providing a promising platform for the treatment of skin aging.8
Vitamin Trojan Horses (Prodrugs)
Trojan horse delivery refers to the chemical modification of an active agent into another ingredient via ionic or covalent bonding. This enhances its skin penetration, and upon entering the skin, the original active is released to provide the intended action. Such chemical modifications, referred to as prodrugs, are well-known in pharmaceuticals although they themselves may or may not have any biological attributes. Prodrugs also offer generally improved stability over the active agent, for which reason their application in the cosmetics is increasing. For example, retinyl (or vitamin A) acetate, a prodrug of retinol, shows 100% retention in skin after 24 hr over retinol.9
Retinyl palmitate has become one of the most popular ingredients in anti-aging skin care products. Limited information is available on its penetration and distribution in vivo in the skin. However, a comparative study of retinol versus retinyl palmitate showed that retinyl palmitate diffused into the skin and was partially hydrolyzed to retinol. Levels of retinyl palmitate in the skin of mice that were administered a retinyl palmitate cream were found to be higher than the control.10
Vitamin E (α-tocopherol), another popular ingredient in anti-aging formulations, is used most commonly in its prodrug form of vitamin E acetate, due to improved stability. A comparison of vitamin E prodrugs with higher chain fatty acids suggested that α-tocopheryl conjugates with unsaturated fatty acids may be more stable derivatives than α-tocopheryl acetate.11
Ascorbic acid (vitamin C) is yet another popular cosmetic ingredient for anti-aging products. It is unstable in water-based formulations, especially in the presence of air and/or light. Ascorbyl esters, most notably ascorbyl palmitate, have become workhorse ingredients in cosmetics due to their availability and favorable cost. In an in vivo study, ascorbyl palmitate showed better antioxidant efficacy than ascorbic acid.12 Ascorbic acid derivatives with improved stability also have become available.13
The prodrugs of vitamins A, C and E described above are mostly covalent-bonded derivatives that require distinct chemical steps in their manufacturing process. Alternate versions of prodrugs, those formed via ionic bonding dubbed ion-pair delivery systems, also have been introduced. These chemical modifications can usually be performed during the manufacturing process of the formula itself. To illustrate, complexes of popular hydroxy acids, i.e., zinc glycinate glycolate and zinc glycinate salicylate (see Figure 3), have been reported to cause less skin irritation due to their favorable pH profile and a dual benefit from both their hydroxy acid and zinc moieties, for skin anti-aging and anti-acne applications.14
Carrier-based Transport
Carrier-based, active agent-loaded vesicular and particulate delivery systems can also provide enhanced skin penetration, compared with conventional emulsions or suspension-based topical delivery systems.15 A discussion of select carrier-based topical delivery systems of current interest follows (see Figure 4).
Nano- and micro-particle carriers: This is the fastest-growing class of delivery systems currently under development for topical pharmaceuticals, with potential application in skin care. These polymeric, solid, multi-compartment delivery systems are promising forms for topical application. Nanoparticles are 10–1,000 nm, whereas microparticles are 1–10 µm. Commonly used nanocarriers include micelles, liposomes, ethosomes, vesicles, microemulsion polymers, carbon-based materials and other substances that are the size of only a few nanometers.16
Inclusion complexes: Structured polymers such as cyclodextrin and zeolites possess a pre-determined molecular gate opening size to permit the entrance and exit of a host molecule. They have been well-known for the delivery of pharmaceuticals. Eye drops, for example, containing drug inclusion complexes, namely dexamethasone or pilocarpine with 2-hydroxypropyl-β-cyclodextrin. These are well-tolerated and ensure increased bioavailability, in comparison with conventional formulations delivered as water and oil solutions, emulsions, or suspensions.17
Macromolecules: The topical penetration of macromolecules into skin is limited by their low permeability. However, they offer potential as a carrier for active agents, such as polyethyleneimine and polyethylene glycol, due to their low toxicity.18
Dendrimers: Dendrimers are branched, spherical, three-dimensional polymer structures of specific size, shape and molecular mass with many functional groups; i.e., carboxyl, hydroxyl and amine. These groups form electrostatic or covalent bonds with an active, allowing dendrimers to carry the active inside their structure. In particular, amine-terminated polyamidoamine (PAMAM) dendrimers (see Figure 5) and their surface modified derivatives have received much recent attention.19 Peptide dendrimers and cell-penetrable glycopeptide dendrimers also have been reported (see Figure 6).20
The ’somes
Various forms of ’somes—i.e., liposomes, niosomes, ethosomes, etc.—are commonly used as delivery systems in cosmetic and skin care formulas. Simply stated, ‘somes are a group of nano- to micro-sized liquid or semisolid carriers stabilized in an immiscible emulsion or suspension liquid medium to transport solubilized active agents in the carrier particles into or across the skin. Nanoparticle and microparticle systems, discussed previously, are based on solid particles and are divergent from ‘somes.
Liposomes: These carriers are composed primarily of phospholipids such as phosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol and α-L-dipalmitoyl-phosphatidylcholine that may additionally contain stearylamine, cholesterol or lecithin. These carriers offer advantages based on their biocompatibility, biodegradability and low toxicity. However, their stability often is of concern, in comparison with delivery systems based on polymers. Their volume for accommodating active agents also can be limited.
Niosomes: Niosomes are two-layered carriers comprised of nonionic surfactants, which are useful for the delivery of both hydrophilic and hydrophobic ingredients.
Ethosomes: These are specially tailored, soft, malleable, vesicular carriers composed of phospholipid, ethanol and water. They can efficiently deliver various molecules with different physicochemical properties into deep skin layers and across the skin. The unique structure of ethosomes enable them to bind to highly lipophilic molecules such as cannabinoids, testosterone and minoxidil, as well as cationic drugs such as propranolol and trihexyphenidil to deliver them through skin.21
Ethosomes have been used mostly for the delivery of lipophilic ingredients, as exemplified above. They also can be bound to molecular-sized peptides, referred to as skin-penetrating and cell-entering (SPACE) peptides (see Figure 7), which are conjugated to phospholipids and used to prepare an ethosomal carrier system. These SPACE-bound ethosomes have been used for the delivery of certain water-soluble and macromolecular ingredients, such as hyaluronic acid and short-interfering RNAs (siRNAs). siRNAs are potential therapeutics for various dermatological diseases including psoriasis, atopic dermatitis and cancer.
The delivery of siRNA poses a challenge due to cellular and tissue level transport barriers, resulting in low absorption across the SC and into viable cells of the skin. In vitro studies revealed that SPACE peptides, when conjugated to other carriers such as small molecules and proteins, were able to facilitate the penetration of siRNAs across the SC into epidermis and dermis. The peptides also exhibited increased penetration into various cells, including keratinocytes, fibroblasts and endothelial cells, likely via a macropinocytosis pathway.
The ability of SPACE peptides to deliver siRNA was also tested in vivo using two targets, interleukin-10 and glyceraldehyde-3-phosphate dehydrogenase. Conjugation of the peptide to siRNA led to their enhanced absorption into skin, and inhibition of corresponding protein targets.22 This discovery opens a new method for selective topical delivery of skin brightening, anti-aging, anti-wrinkle, antioxidant and other skin benefit agents.
Discosomes: These are modified forms of niosomes made from a mixture of ethoxylated cholesterol and ethoxylated fatty alcohols.
Unconventional Delivery Technologies
Conventional transdermal delivery is limited to methodologies wherein the active agent can diffuse across the skin barrier. By this broad definition, the active agent is passed through an artificial route into the body undistorted, without being passed through the body’s various defense systems; for example, being degraded in the gastrointestinal tract or metabolized in the liver. With current delivery methods, successful transdermal active agents have a molecular weight of up to 800 Daltons, which heavily favors lipophilic agents. It has been difficult to exploit the transdermal route to deliver hydrophilic agents, peptides and macromolecules, including new genetic treatments employing DNA or siRNA.
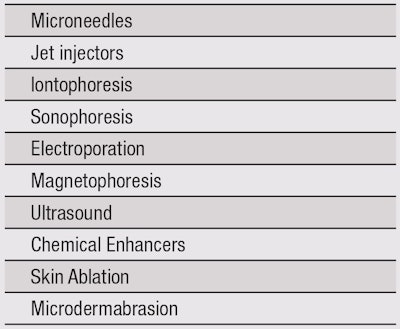
A number of alternate, minimally invasive topical delivery technologies have also been developed, as listed in Table 1. However, this article has focused on formulation-driven delivery systems that are relatively non-invasive to the SC. Any detailed description of these unconventional systems is beyond the scope of this article, and review articles are available elsewhere.4
Regarding formulation-based unconventional delivery technologies, a few include the following.
Salicylic acid: The formulation of hydroxy acids, including salicylic acid, via anhydrous delivery systems has been discussed.23
Retinaldehyde: Retinaldehyde cyclodextrin hemiacetal and acetal complexes for topical delivery of stabilized retinaldehyde have recently been described (see Figure 8).24
L-Ascorbic acid (vitamin C): This potent collagen-boosting, wrinkle-reducing and skin brightening antioxidant is instable in water-based formulations exposed to air and/or light. Ascorbic acid has been formulated using a plethora of methods, including anhydrous preparations, liposomes, prodrugs,25 entrapment and microencapsulation to circumvent these issues.
Summary
A beneficial skin care ingredient must travel across the uppermost layers of the skin to reach targeted cells in the layers below to deliver the desired attributes; although it is a natural function of the uppermost layers of skin to hinder this movement of ingredients. The delivery systems described in this article facilitate this extracellular transport function without imparting undesirable affects either to the skin or to its tactile or biological functions. A variety of delivery systems based on a host of physical or biological mechanisms have been developed to date to answer this dilemma. This pursuit is likely to continue into the distant future, as no single delivery system may be able to perform as a universal antidote to skin’s barrier functions.
References
- A Kubo et al, The stratum corneum comprises three layers with distinct metal-ion barrier properties, Sci Rep 3 1731, doi: 10.1038/srep01731 (Accessed Aug 11, 2014)
- P Garidel, B Fölting, I Schaller and A Kerth, The microstructure of the stratum corneum lipid barrier: Mid-infrared spectroscopic studies of hydrated ceramide:palmitic acid:cholesterol model systems, Biophys Chem 150(1–3) 144-56, doi: 10.1016/j.bpc.2010.03.008 (Aug 2010)
- CM Weiss, Conventional topical delivery systems, Dermatol Ther 24(5) 471–6, doi: 10.1111/j.1529-8019.2012.01458.x (Sep/Oct 2011)
- MR Prausnitz and RR Langer, Transdermal drug delivery, Nat Biotechnol 26(11) 1261–1268, doi: 10.1038/nbt.1504 (Nov 2008)
- G Oliveira, J Hadgraft and M Lane, The influence of volatile solvents on transport across model membranes and human skin, Int J Pharm 435(1) 38–49, doi: 10.1016/j.ijpharm.2012.05.037, epub May 24, 2012 (Oct 2012)
- www.ncbi.nlm.nih.gov/pubmed/24798887 (Accessed Aug 11, 2014)
- www.ncbi.nlm.nih.gov/pubmed/24797876 (Accessed Aug 11, 2014)
- www.ncbi.nlm.nih.gov/pubmed/24772430 (Accessed Aug 11, 2014)
- A Arayachukeat, SP Wanichwecharungruang and T Tree-Udom, Retinyl acetate-loaded nanoparticles: Dermal penetration and release of the retinyl acetate, Int J Pharm 404(1-2) 281–8, doi: 10.1016/j.ijpharm.2010.11.019, epub Nov 18, 2010 (Feb 14, 2011)
- J Yan et al, Levels of retinyl palmitate and retinol in the skin of SKH-1 mice topically treated with retinyl palmitate and concomitant exposure to simulated solar light for thirteen weeks, Toxicol Ind Health 23(10) 581–9 (Nov 2007)
- S Ben-Shabat, Y Kazdan, E Beit-Yannai and AC Sintov, Use of alpha-tocopherol esters for topical vitamin E treatment: Evaluation of their skin permeation and metabolism, J Pharm Pharmacol 65(5) 652-8, doi: 10.1111/jphp.12027, epub Jan 25, 2013 (May 2013)
- D Gopinath, D Ravi, BR Rao, SS Apte, D Renuka and D Rambhau, Ascorbyl palmitate vesicles (Aspasomes): Formation, characterization and applications, Int J Pharm 271(1–2) 95-113 (Mar 1, 2004)
- AI Segall and MA Moyano, Stability of vitamin C derivatives in topical formulations containing lipoic acid, vitamins A and E, Int J Cosmet Sci 30(6) 453–8, doi: 10.1111/j.1468-2494.2008.00473.x (Dec 2008)
- US Pat 7,547,454, Zinc glycinate glycolate; topical use treating skin disorders, wrinkles, liver spots, dry skin, xerosis, ichthyosis, blemishes, assigned to S Gupta (Jun 16, 2009)
- www.hindawi.com/journals/tswj/2014/276260/ (Accessed Aug 11, 2014)
- www.dovepress.com/ph-sensitive-strontium-carbonate-nanoparticles-as-new-anticancer-vehic-peer-reviewed-article-IJN (Accessed Aug 11, 2014)
- www.ncbi.nlm.nih.gov/pubmed/24772038 (Accessed Aug 11, 2014)
- www.ncbi.nlm.nih.gov/pubmed/?term=10.1016%2Fj.ijpharm.2013.08.001 (Accessed Aug 11, 2014)
- www.ncbi.nlm.nih.gov/pubmed/?term=10.1016%2Fj.addr.2011.09.010 (Accessed Aug 11, 2014)
- J Zhao e tal, Cell-penetrable lysine dendrimers for anti-cancer drug delivery: Synthesis and preliminary biological evaluation, Arch Pharm (Weinheim), doi: 10.1002/ardp.201300415 (Apr 17, 2014)
- www.ncbi.nlm.nih.gov/pubmed/12911264 (Accessed Aug 11, 2014)
- www.ncbi.nlm.nih.gov/pubmed/?term=10.1016%2Fj.jconrel.2013.10.007 (Accessed Aug 11, 2014)
- S Gupta, Hydroxy acids delivery system, ch 6 in Skin Delivery Systems, JJ Wille, ed, Blackwell Publishing, Hoboken, NJ (2006)
- US Pat 8,410,079, Chirally correct retinal cyclodextrin hemiacetals for clarifying skin complexion, DW Peter et al, assigned to Island Kinetics (Apr 2, 2013)
- US Pat 7,842,723, Ascorbic acid—Natural sugar lactone esters for comprehensive skin and scalp care, S Gupta, assigned to Bioderm Research (Nov 30, 2010)