Over the years, the personal care industry has experienced many changes; perhaps none more profound or more justified than accepting increasing responsibility for the impact of its products on the environment. In fact, understanding and accepting the concepts of stewardship is a critical step in new product development.
See related sidebar: Synthetic Membrane for Non-animal Transdermal Diffusion Testing [sponsored]
As described by the ACTA consulting firm, which assists in making and using sustainable chemicals globally, product stewardship refers to the safe and effective management of chemicals throughout their lifecycle.1 “This is not only environmentally responsible, but is also increasingly requested by the public and required by governments worldwide,” the firm’s site explains.
“To address public concern, governmental pressures and competitive demands, participants in the chemical manufacturing process must be proactive in managing and monitoring their products to minimize risk and safeguard the public from health and environmental hazards. Developing, maintaining and helping to evolve responsible product strategies are critical to the success of companies in the chemical space.”
The environmentally conscious movement has roots in the 1950s, which led to the culmination and publication of a book key to this phenomenon, written by Rachael Carson,2 entitled Silent Spring.3 Wikipedia states, “Silent Spring is an environmental science book published on September 27, 1962, documenting the adverse environmental effects caused by the indiscriminate use of pesticides. Carson accused the chemical industry of spreading disinformation, and public officials of accepting the industry's marketing claims unquestioningly. The result of her research was Silent Spring, which brought environmental concerns to the American public.”
Despite the fact that we have made many advances in our understanding of environmental issues, including the establishment of the U.S. Environmental Protection Agency (EPA)—which is chartered to protect people and the environment from significant health risks; to sponsor and conduct research; and to develop and enforce environmental regulations—there remains work to be done.
Properties in addition to those envisioned at the time Silent Spring are now being evaluated. These can be divided into four basic properties of an ingredient or raw material: 1) biodegradable, 2) sustainable, 3) readily commercially available and 4) green. These properties serve as the basis for the present discussion.
To address these topics with specificity, there must be an accepted definition, accepted testing methodologies, and accepted approaches to evaluate what this category means to the consumer, who after all, is the final decision maker in the personal care market. All raw materials used in the personal care industry can be modified to improve the values obtained for each of these categories by chemical modification; i.e., by placing groups within the molecule to improve the property desired.
As Mellou, et al., explain, “A global tendency for products considered environmentally sustainable and ecologically obtained [has] led the industry related to personal care formulations to fund the research and the development of personal care/cosmetics containing ingredients from natural resources. Furthermore, consumers are aware of environmental and sustainability issues…thus, not harming the environment represents a key consideration when developing a new cosmetic ingredient.”4
Biodegradable
Biodegradation, i.e., biotic degradation or biotic decomposition, is the chemical degradation of contaminants by bacteria or other biological means, and is often referred to as the natural method of degrading discharged chemicals. Most biodegradation systems operate under aerobic conditions but a system under anaerobic conditions may permit microbial organisms to degrade chemical species that are otherwise nonresponsive to aerobic treatment, and vice versa.
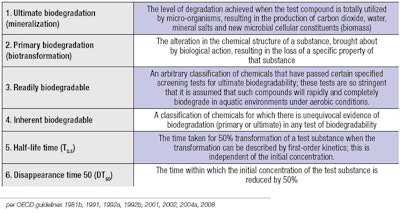
Thus, biodegradation is a natural process or series of processes by which chemicals or other waste materials can be broken down or degraded into nutrients that can be used by other organisms. The ability of a chemical to be biodegraded is an indispensable element in understanding the risk posed by that chemical in the environment. Biodegradation is a key process in the natural attenuation, reduction or disposal of chemicals.5 Table 1 outlines the Organization for Economic Cooperation and Development’s (OECD’s) guidelines distinguishing six forms of biodegradation:6 ultimate biodegradation, primary degradation, readily biodegradable, inherently biodegradable, half-life time (T0.5), and disappearance time 50 (DT50). The full report on biodegradation is available online.7
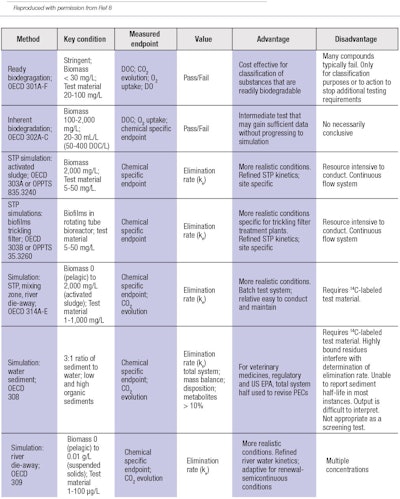
Table 2 outlines recommendations for biodegradation testing.8 Upon review, three important factors are clearly required to successfully navigate the analysis:
1. Diversity of tests. A number of different tests must be available for potential selection and use.
2. Specification of the test. The test chosen for the evaluation must be identified.
3. Identification of the percentage of actives to be tested.
Additional terms used for products to describe their environmental fate include degradation for non-bio-based and hydrolyzable, and these also must be considered. An example of test data is shown in Figure 1 and Figure 2 (figures and data courtesy of Covestro). The ability to review such data, as well as conclusions drawn from the data, is critical to understanding and supporting conclusions about it.
The article9 related to Figure 1 and Figure 2 goes on to say: “Natural polymers are assumed by consumers to biodegrade better. However, just as for synthetic substances, the level and kinetics of biodegradation may strongly vary depending on the chemical structure of the polymer. Furthermore, natural polymers are often modified for the purposes of the application, which may significantly impair their biodegradability. This is sometimes the case with cellulose, which, when modified into carboxymethyl cellulose or hydroxyethyl cellulose, loses its ability to biodegrade with increasing degree of modification.”
Another factor to consider is the percentage of the raw material in a blend that is being tested. If a non-biodegradable product is being tested in a biodegradable solvent, the biodegradation conclusion may well be very different if that same material is tested neat. Again, proper definitions and conditions are critical.
Sustainable
Sustainable is defined as capable of being maintained at a steady level without exhausting natural resources or causing severe ecological damage. Sustainable chemistry refers to the scientific concept that seeks to improve the efficiency with which natural resources are used to meet human needs for chemical products, services, etc. Some environmental and social benefits of sustainable chemistry are outlined in Table 3.10
According to The Free Dictionary, “Sustainable chemistry encompasses the design, manufacture and use of efficient, effective, safe and more environmentally benign chemical products and processes." It is also, “a process that stimulates innovation across all sectors to design and discover new chemicals, production processes and product stewardship practices.” These, in turn, aim to increase performance and value while also protecting and enhancing human health and the environment.10
With the concept of sustainability comes the concept of cosmetic ethics. The cosmetic chemist is responsible for developing products to be used by consumers on their person in order to achieve numerous benefits. In addition to cosmetics are several over-the-counter products classified as drugs that formulators are called upon to develop; i.e., dandruff shampoos, antiperspirants and sun care products. The formulation of these products not only requires the product to be safe and to provide the benefits claimed, but also the chemist to responsibly choose raw materials and create formulations that are consistent with our obligations to the environment; i.e., they must not have an adverse impact on our planet or cause unintended consequences.
Considering these facets of product development, some higher-level and even philosophical questions to ask include the following.
- In a world where ongoing hunger and starvation are realities, should we as an industry be using edible oils, e.g., olive oil, to make cosmetic products while inedible raw materials such as ethylene-based products are available?
- In attempt to protect coral reefs, are we trading off human protection? The organic sunscreens of alleged concern tend to be the most effective, so discontinuing their use could mean a rise in human skin cancer rates. Also, what responsibility does the U.S. Food and Drug Administration have in expediting the approval of European-approved sunscreens that could address this?
- Is it acceptable to use non-biodegradable materials, e.g., plastics or acrylates, in formulations knowing they are accumulating in our oceans? Does the fact that these materials “do no harm” make any difference?
The concept of sustainability has many additional implications. Not only does it relate to renewable, i.e., materials capable of being maintained at a steady level without exhausting natural resources or causing severe ecological damage, but it also relates to the intersection environmental, economic and community concerns; such a Venn diagram is shown in Figure 3.11
Metrics for sustainability are continually developed. For example, carbon footprints are widely used but will likely be extended to consider energy and water consumption, as well as waste and social parameters.12
Readily Commercially Available
In recent years, there have been instances where strategic intermediates have experienced either a lack of supply or sharp price increase. Sometimes these price increases last a short term; sometimes they are longer term. Products based upon raw materials that are subject to wide swings in price are not suitable for use in a long-term product. As such, it is important to note they will simply not find practical application in sustainable products.
Greenness of Chemistry
Finally, in relation to a material’s biodegradation and eco-safety is the greenness of its chemistry. U.S. patent application 20090259409 describes a “process for determining the Green Star rating of compounds and formulations,” and teaches a process that can be used in the formulation of more environmentally friendly, greener formulations for consumer applications.
The process involves evaluating components in a formulation, then determining the percentage of the molecule that is green. This establishes a Green Star rating and determines the effect of that component on the overall Green Star rating of a formulation.13
This rating ultimately provides a process by which a formulator can easily ascertain the greenness of a raw material and, as importantly, a consumer can determine and compare the greenness of a formulation to similar types of products. This process provides the formulation chemist with a way to break a molecule down into its green portion and non-renewable resource portion.14
In determining the “green” portion of the molecule, C13 NMR is commonly the method of choice. As described in the patent, “Carbon is a naturally abundant element found in the atmosphere, in the earth, in the oceans and in every living creature. C-12 is by far the most common isotope, while only about one in a trillion carbon atoms is C-14. C-14 is produced in the upper atmosphere when nitrogen-14 (N-14) is altered through the effects of cosmic radiation bombardment (a proton is displaced by a neutron, effectively changing the nitrogen atom into a carbon isotope).
“The new isotope is called ‘radiocarbon’ because it is radioactive, though it is not dangerous. It is naturally unstable and so it will spontaneously decay back into N-14 after a period of time. It takes about 5,730 years for half of a sample of radiocarbon to decay back into nitrogen. It takes another 5,730 for half of the remainder to decay, and then another 5,730 for half of what's left then to decay, and so on. The period of time that it takes for half of a sample to decay is called a ‘half-life.’”14
Carbon dating is a technique predicated upon three things:
- The rate at which the unstable radioactive C-14 isotope decays into the stable non-radioactive N-14 isotope;
- The ratio of C-12 to C-14 found in a given specimen; and
- The ratio of C-12 to C-14 found in the atmosphere at the time of the specimen's death.”15
Having an accurate method to determine the age of the “carbon” in a product allows for a determination of age and thereby the green nature of the raw materials.
Conclusions
The words biodegradable, sustainable, available and green are, first and foremost, technical terms to describe the key characteristics of ethical personal care; they are marketing terms secondarily. The problem is, despite the fact that they have been used for years, they still lack complete specificity.
Add to this the fact that a number of different test methodologies can be employed, and that the actual results of tests or specific methods used may not be reported. The personal care industry not only needs to standardize the methods chosen to support these claims, but also to publish more data on the methods used and the results obtained. This will ensure personal care products are created that support these tenets and are formulated in good conscience.
Got Biodegradation Data?
As a final appeal to the industry, the author and editors at Cosmetics & Toiletries invite you to submit biodegradation information and data on various classes of compounds to help us compile a resource for all to use. Send submissions on ethoxylates/propoxylates, acrylates, esters and branched esters, silicones (PEG/PPG dimethicone), sulfates, cationics and more to [email protected].
References
All websites accessed on Feb. 4, 2020.
- ACTA. Chemical product stewardship. Retrieved from https://www.actagroup.com/index.php/practices/chemical-product-stewardship
- The Life and Legacy of Rachel Carson. Retrieved from https://rachelcarson.org
- Wikipedia. Silent Spring. Retrieved from https://en.wikipedia.org/wiki/Silent_Spring
- Mellou, F., Varvaresouand, A. and Papageorgiou S. (2019, Aug 1). Renewable sources: Applications in personal care formulations. Intl J Cos Sci. Retrieved from https://doi.org/10.1111/ics.12564
- ˇZupunski, M., ... Boriˇsev, M., et al. (2018). Insights and lessons learned from the long-term rehabilitation of abandoned mine lands—A plant based approach. Chemical Degradation. Retrieved from https://www.sciencedirect.com/topics/earth-and-planetary-sciences/chemical-degradation
- ECETOC. Technical report 123. Definition(s) according to OECD. Retrieved from http://www.ecetoc.org/report/measured-partitioning-property-data/biodegradation/definitions-according-to-oecd/
- OECD. (2005, Apr). OECD guidelines for testing of chemicals. Retrieved from http://www.oecd.org/chemicalsafety/testing/34898616.pdf
- ECETOC. Technical report 123. Summary and Recommendation. Retrieved from http://www.ecetoc.org/report/measured-partitioning-property-data/biodegradation/summary-and-recommendation/
- Pottié, L. (2019, Feb 5). Getting the breakdown: Testing the biodegradation and styling performance of polyurethane film formers. Cosm & Toil. Retrieved from https://www.cosmeticsandtoiletries.com/testing/toxicityanalysis/Getting-the-Breakdown-Testing-the-Biodegradation-and-Styling-Performance-of-Polyurethane-Film-Formers-505373421.html
- The Free Dictionary. Sustainable. Retrieved from www.thefreedictionary.com/Sustainable
- OECD. Sustainable Chemistry. Retrieved from http://www.oecd.org/chemicalsafety/risk-management/sustainablechemistry.htm
- Whaung Pei Hwee, L. (2014, Jul 17). The difference between green & sustainable. Integrated Project, Matilda Street blog. Retrieved from http://b3044005lyvia.blogspot.com/2014/08/the-difference-between-green-sustainable.html#comment-form
- Whitehouse, L. (2017, Feb 7). Sustainable cosmetics in 2017: What does the year ahead hold? Cosmetics Design-europe.com. Retrieved from https://www.cosmeticsdesign-europe.com/Article/2017/02/07/Sustainable-cosmetics-in-2017-what-does-the-year-ahead-hold
- O’Lenick, A. (2009, Oct 15). Process for determining the green star rating of compounds and formulations. U.S. Pat App 20090259409. Retrieved from http://www.patentsencyclopedia.com/app/20090259409
- O’Lenick, A. and O’Lenick, K. (2011 Mar). Green rating for compounds and formulations. Personal Care. Retrieved from http://www.scientificspectator.com/documents/Olenick%20Compilation/Ch%2036%20Green%20Star.pdf