The Association of Southeast Asian Nations (ASEAN) has agreed to harmonize its cosmetic regulatory scheme. This decision went into effect in January 2008. The 10 nations that are part of the ASEAN group include Brunei , Cambodia, Indonesia, Laos, Malaysia, Myanmar, the Philippines, Singapore, Thailand and Vietnam(see Table 1).
The harmonization into a single Cosmetic Directive has the benefits of enhanced cooperation between the countries, eliminating trade restrictions, and ensuring safety, quality and claimed benefits for all products. This scheme was agreed upon in 2003 and planned to take effect in 2008. The agreement consists of seven articles, two schedules and seven lists.
Article 1 states the objectives of ensuring safety, quality and claims, and was established to eliminate trade restrictions of cosmetics through harmonization of technical requirements and mutual recognition of registration and approvals. Article 2 states that the members will adopt these regulations by Jan. 1, 2008, and Article 3 lists the technical documents required for cosmetics,including:
- a definition and illustrative list of cosmetics,
- an ingredient listing and handbook of ingredients,
- labeling requirements,
- claims guidelines,
- registration requirements,
- import/export requirements, and
- guidelines for good manufacturing practices.
Article 4 states that members should strengthen and enhance cooperation in areas not already covered, and Article 5 adds that any differences should be resolved by negotiation between members. Article 6 establishes an ASEAN Cosmetic Committee (ACC) consisting of one representative of the cosmetics regulatory authority for each member state. The ASEAN cosmetics industry such as those involved in the ASEAN Cosmetic Association (ACA) trade organization, will be invited to attend ACC meetings and will be consulted on all matters concerning the industry. The ACC also will review and update the technical documents listed in Article 3 and can consult outside bodies for advice. Finally, Article 7 lists the final provisions regarding how the harmonized regulations of cosmetics will be approved.
Schedule A
Schedule A is divided into five articles for cosmetic product registration approvals. Article 1 states that each member state will recognize registrations from other states, resulting in a product that need be registered only once. Article 2 adds that only registered cosmetics can be marketed in other states.Under Article 3, a notification letter informing the other states of information found on Appendix IV and a certified copy of the Certificate of Registration are required. Within 30 days, the other states must indicate that either the cosmetic is allowed or present information as to why it is being disallowed. In Article 4, member states are encouraged to participate in the procedure described in Article 3. In addition, Article 4 states that two or more member states can proceed even if other states are not ready. This was intended to allow regulations to move forward even though all countries have not changed their regulations to conform to the new regulations. Finally, Article 5 states that any member state may withdraw from or choose not to be part of the ASEAN cosmetics regulations so long as providing three months’ notice.
Schedule B
Schedule B outlines the ASEAN Cosmetic Directive that consists of 12 Articles.
Article 1—General Provisions: Member states will take all measures to ensure that only cosmetics that comply with the rules are allowed on the market. If a product complies, it must be allowed on the market. The company or person who places the product on the market is responsible for completing the proper notifications, including the location of manufacture and initial import, before the product is placed on the market. This company or person will serve as keeper of the technical and safety information and must have this information readily accessible to the individual regulatory authorities of the member state.
Article 2—Definition and Scope of a Cosmetic Product: This article includes the ASEAN definition of a cosmetic. The group has used the European Union’s (EU’s) definition, as found in the EU Cosmetic Directive 76/768/EEC Article 1; 1. A.
Article 3—Safety Requirements: Cosmetics must not cause damage to human health under normal or reasonably foreseeable conditions of use. This fact should be taken into account on labeling, in instructions, during disposal, and in warnings. Warnings do not exempt products from compliance with this directive.
Article 4—Ingredient Listings: This article is rather complex and broken into several categories:
1. The ASEAN group has adopted the EU’s list of approved ingredients.
2. Ingredients that are prohibited include:
- Substances listed on Annex II of the EU Directive
- Substances listed on Annex III, part A, of the EU Directive that exceed the conditions of Annex III
- All colors must be listed on Annex IV of the EU, with the exception of colors for use as hair dyes only
- All colors listed on Annex IV and used outside of the conditions outlined in Annex IV of the EU, with the exception of colors for use as hair dyes only
- Preservatives other than those listed on Annex VI of the EU Cosmetic Directive
- Preservatives listed in Annex VI and used outside of the conditions outlined in Annex VI of the EU Cosmetic Directive
- UV filters other than those listed in Annex VII of the EU Cosmetic Directive
- UV filters listed in Annex VII and used outside of the conditions outlined in Annex VII of the EU Cosmetic Directive
3. The presence of trace substances listed in Annex II shall be allowed provided they are technically unavoidable in good manufacturing practices (GMP).
4. Member states shall allow cosmetics containing substances in Annex III, Annex IV, Annex VI and Annex VII, the second part of each, of the EU Cosmetic Directive and used within their conditions and dates of permitted use.
At the given dates, the ingredients must be definitively allowed, prohibited, maintained for a specific period of time if the EU extends the part two listing, or deleted from the annexes as no longer being used.
Article 5—ASEAN Handbook of Cosmetic Ingredients: In addition to Article 4, member states may allow the use, within their country, of substances for up to three years providing they conduct an official investigation on the cosmetics made with the substance, and that the product bears a distinctive indication that will define its authorization. Before the end of the three-year period, the member state may submit a request for inclusion in the permitted lists to ACC.
Article 6—Labeling: Product labeling is defined in Appendix II. Labels should include the product name and function, a full ingredient listing, contents, a manufacturing or expiration date if the product has a stability of less than 30 months, the name and address of the company responsible for placing the product on the market, instructions for the product’s use, the country of manufacture, a batch or lot number, and special precautions from the annexes or as determined by the company.
Article 7—Product Claims: All product claims must comply with the ASEAN Cosmetic Claims Guidelines listed in Appendix III. The claims can be substantiated by the testing of the formulation itself. The data must be kept available in the same location as the safety and technical data.
Article 8—Product Information: At the address indicated on the product label, the following information must be accessible to the authorities:
- The qualitative and quantitative composition of the product, name and code, the fragrance included in the product and idenitity of the supplier of the perfume.
- Specifications of raw materials and the finished product
- The method of manufacture and indication of compliance with GMPs as written in ASEAN guidelines; the person responsible for manufacture or importation must have knowledge of the local laws or experience with the local legislation.
- Safety assessments of the cosmetic product and ingredients, chemical structure of ingredients, and level of exposure
- Existing data on undesirable effects to human health
- Supporting data for claims
The above information must be listed in the national language of the specific country or a language understood by the regulating authority. For medical reasons, the member state may require information about the ingredients for treatment purposes.
Article 9—Method of Analysis: The following also must be available to the authorities: methods used during manufacturing to confirm ingredient certificates of analysis and the criteria used to establish microbiological control of cosmetics and chemical purity of ingredients.
Article 10—Institutional Arrangements: The ACC shall review and monitor the implementation of the ASEAN Cosmetics Directive. The group may establish a committee for Standards and Quality (ACCSQ) and a Scientific Body (ACSB) to assist ACC in the review of technical issues. The ACSB shall consist of representatives from regulatory authorities, industry and academia.
Article 11—Special Cases: Member states may provisionally prohibit a cosmetic product if there is a substantiated justification, such as a health hazard or religious or cultural sensitivity. Certain product claims may also be prohibited. The member state must promptly inform the other states and the ASEAN Secretariat stating the reasons.
Article 12— Implementation: Member states must change their local laws to comply with the newly established regulations and send copies to the other states and the ASEAN Secretariat. Member states must allow the marketing of noncompliant products for up to 36 months if the products were placed on the market before Jan. 1, 2008.
Appendix II: Cosmetic Labeling Requirements
Article 6 from above references Appendix II for labeling of products. The following must be included on the outer packaging:
- the name of the cosmetic and its function, unless it is clear from the name;
- instructions for use unless it is clear from the name;
- The full ingredient listing in descending order to 1%; items below 1% may be listed in any order. Colors can be listed last using CI numbers. The use of “may contain” or the symbol “+/-” is permitted for decorative products marketed in several shades. Ingredient names should be taken from the latest edition of the INCI Dictionary, British Pharmacoepia, US Pharmacoepia or Chemical Abstract Services. Botanicals and extracts should be listed by genus and species, and the abbreviation of the genus is permitted.
- Country of origin;
- The name and address of the company or person placing the product on the market;
- Net contents in metric or both metric and imperial systems;
- The manufacture’s batch number;
- Expiration date; and
- Special precautions for use.
In cases where there is not enough room for all of this information, manufacturers can use leaflets, pamphlets, hang tags, display panels or shrink wrap. The package must include the name and batch number and information on the product package should be listed in English and/or the national language that the product is sold in.
Appendix III: ASEAN Cosmetic Claim Guidelines
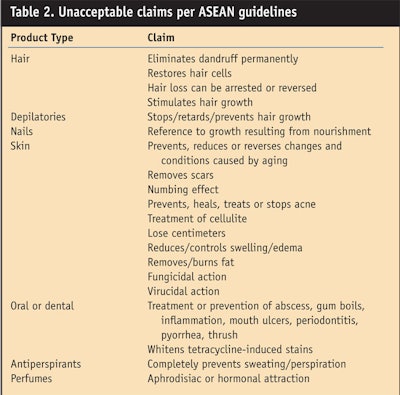
Following are the guidelines for claims. The ASEAN guidelines list the following claims as secondary: dandruff, cellulite, bust contouring, antibacterial, caries, hair loss, acne and mouthwash. Table 2 lists examples of unacceptable claims.
Claims can be made more acceptable by modifiers such as: helps to remove oil from skin, suitable for oily skin, reduces shine of oily skin, or makes skin feel less oily.
Discussion
The new ASEAN rules may be considered as “EU light.” They follow many EU regulations with some key differences. Manufacturers must notify each member state, whereas in the EU, manufacturers must notify the country whose address is on the label. Under the ASEAN regulations, manufacturers must list the country of origin, whereas the EU requires this information only for products from outside of the EU.
It is useful that under ASEAN regulations, prohibited ingredients are listed and maintained in alphabetical order, unlike the EU, which lists them in order of addition to the list. However, the ASEAN rules continue the EU’s practice of banning over 1,200 ingredients, of which maybe 12 have ever been used in cosmetics.
The notation permitting dual net contents on the label is not surprising since Myanmar, along with Liberia and the United States, are the only countries in the world still using the old imperial system of weights and measurements. Finally, it is promising that industry members are allowed to participate on the scientific committee. Maybe someone who has actually made a cosmetic will be present to help make critical decisions, unlike the EU’s Scientific Committee on Consumer Products.