While the industry and general public are highly educated about the dangers associated with excessive exposure to sunlight, especially the UVA and UVB wavelengths, findings in recent years reveal that the full spectrum of benefits and harm related to sun exposure are yet to be explored. One of the more striking recent discoveries is that while UVA and UVB are indeed responsible for skin damage, high energy visible (HEV) light, i.e. in the violet and blue range, may cause as much damage as UVA and UVB combined.1–4 However, developing a compound to shield the skin by the selective filtration of violet and blue light presents challenges in molecular design as well as formulation because an effective compound must exhibit color.
UV absorbers in skin care formulas do not impart color because the retina does not respond to the range of photon energies they absorb. However, since HEV absorbers selectively reduce, for example, violet and blue light from the visible spectrum, the eye perceives this as a change in color as the remaining spectrum is expressed; in this case, it appears brown or yellow-brown.
In this paper, the authors present the rationale behind the development of a fractionated melanin (FM) that is tailored to absorb light in the HEV blue to violet wavelength range of 400–500 nm, with minimal absorption in the red range. Since it provides UV as well as HEV photoprotection, it imparts color to formulations. Therefore, the present paper also suggests approaches to cope with the aesthetic challenges this molecule presents when introduced into semi-solid formulations. Overall, this material is presented as a novel means to maximize photoprotection and phototherapy while minimizing its color footprint.
HEV Light and Skin
HEV light is in the blue to violet band, 400–500 nm, of the visible spectrum. The effects of HEV light on macular degeneration have been studied and results suggest it may be a key factor in this age-related disorder.5 The mechanism by which the light damages the lens and retina of the eye is believed to be the generation and accumulation of reactive oxygen species (ROS) that lead to oxidative damage to cells and their organelles. These changes are irreversible and should therefore be minimized as much as possible. In relation to skin, two independent studies were conducted to evaluate the effects of HEV light, which demonstrated photodegradative effects similar to those found in the eye to the epidermal and dermal tissues.
Animal model: Researchers studied the effects of blue light (450–500 nm) on the tape-stripped skin barrier of hairless mice first and discovered a significantly delayed barrier recovery rate in comparison with the control. Notably, green light had no effect, while red light increased the recovery rate.1 This barrier recovery rate was measured by transepidermal water loss (TEWL)—a common practice to assess cutaneous barrier function in a quantitative manner. This technique is based on the fact that when the stratum corneum (SC) is compromised, the rate of water loss from the skin is accelerated.2
In the same study, cultured sections of hairless mouse skin were exposed to HEV wavelengths and electron microscopy evaluation revealed that irradiated skin showed a different morphology from skin kept in the dark (control). The exposed skin demonstrated depleted intercellular lipid content between the SC and the stratum granulosum (SG), suggesting that exposure to HEV light inhibits the processes that support barrier recovery. Further, it is interesting to note that while UVB light penetrates to the live epidermis and UVA penetrates deeper into the dermis, visible light penetrates even deeper than UVA—beyond the dermis and into the circulatory system, passing through and damaging both the epidermis and dermis.3
Ex vivo human model: In a second study, the effects of visible light with and without UVA and UVB on the generation of ROS were studied in an ex vivo human skin model.4 To quantify free radical generation, electron paramagnetic resonance (ESR-X) band spectroscopy was utilized. This method entails analyzing the magnetic moment of unpaired spinning free electrons to detect free radical formation under the influence of all wavelengths—from the UVB range to the end of the visible spectra (280–700 nm). The findings were surprising and revealed that the percentage of radical formation caused by visible light exposure was highly significant. When calculated and normalized to the spectra of exposure to natural sunlight, the results showed that while UVB generated 4% of ROS, and UVA 46%, visible light accounted for 50% of ROS production.
Further identification showed that the radicals produced were the superoxide anion radical O2•– and the hydroxyl radical OH•. It is known that generation of these two highly reactive species can lead to the formation of other biological radicals including secondary lipid radicals, •CH-R, in the different skin layers.6 Such processes of ROS production are well-known to be involved in premature skin aging and are often accompanied by inflammatory cascades, DNA damage, effects on the collagen and elastin network, and depleted immunity.
UV Protection in Personal Care
The cosmetic and personal care industry has thus far focused on the improvement of sun protection formulations to efficiently shield the skin from exposure to UVB and UVA exclusive of any considerations to protect from the visible spectrum of wavelengths. Perhaps this is because an awareness of the effects of visible light on skin is not yet established in the industry, since more studies are required to better understand its chronic and cumulative effects, or because, as noted, in order to protect the skin from visible light, a light-filtering agent of a dark color would most likely be required—and incorporating a dark-colored HEV filter into a skin care product presents an esthetic challenge.
Considering this new skin care target and the formulation challenges posed, a melanin derivative synthesized from vegetable tyrosine was developed to protect skin from HEV light. Melanin has already been applied to sunglasses to shield the eyes from blue light to prevent macular degeneration, which was the inspiration behind this concept. Further, the melanin was fractionated (see Figure 1) to produce smaller polymers for easier application and to increase HEV filtration while minimizing the perception of darkness.
Rationale and Compound Development
FM was specifically designed to shield the skin with maximum absorption of HEV light, and to facilitate repair of the skin with minimal absorbance of red and near infrared (NIR) because light in this region of wavelengths is known to be phototherapeutic to the skin and provide anti-aging benefits. Chemists and physicists have long been familiar with the so-called particlein- the-box concept of de-localized electronic systems, in which smaller molecular weight, conjugated molecules have less absorption in the longer wavelengths—those the eye associates with red color. Since recent science has suggested a range of molecular weights for melanin, an opportunity was presented to fractionate standard melanin and thereby isolate a melanin fraction with less red and NIR absorption and more HEV light absorption.
Research has shown that exposing dermal fibroblasts to red light elevates the levels of collagen and elastin content in the skin,7 although this mechanism is not fully understood. Moreover, the same study describes a clinical study of 20 volunteers who were exposed periodically to red light for six months and found that 51–75% of the participants reported significant improvement in skin texture and color with no effect on pigmentation.
The design of a substance with sufficiently small molecular weight that shields the skin from the harmful wavelengths but allows the passage of beneficial wavelengths was therefore crucial. On the other hand, the FM was also designed to have a sufficiently large molecular weight, estimated to be 5,000–10,000 Daltons, so as to not penetrate the skin. Interestingly, safety studies conducted in vitro revealed that this melanin may also exhibit a cellular protective effect due to its radical scavenging properties.8
HEV Absorbance
To determine the ability of FM to absorb HEV light, a spectrophotometer was used to assess its capability to absorb in the blue range. Figure 2 shows a graphical representation of the performance of FM in the HEV wavelength region, in comparison with standard, full molecular weight melanin. The absorption spectra shown were normalized so that the two curves crossed at around 500 nm. Readers should note that the actual absorption values will vary with the concentration of melanin used. However, the curves can be made to cross at any wavelength desired by multiplying the FM spectrum by an appropriate number.
The spectra show the optical absorption values at the different wavelengths of the visible spectrum. At the red end, between 600 nm and 780 nm, both melanins showed relatively low absorption; however FM absorbed less of this beneficial red light than the standard melanin. Between 400 nm and 500 nm, FM absorbed significantly more of the HEV light than the standard melanin. At 550 nm, the wavelength at which the human eye is most sensitive to visible light,9 FM appears lighter than the standard melanin because it absorbs less light.
Formulating with FM
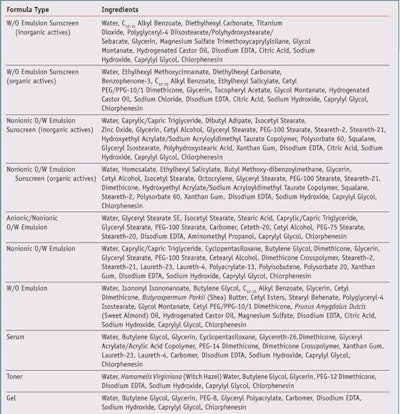
Since FM showed the ability to absorb radiation in the visible blue/violet range, it was then formulated into a selection of common emulsion, sunscreen, and other cosmetic formulations (see Table 1) and compared with formulas omitting FM in order to explore its physical, chemical or aesthetic effects on each formula type. Readers should note that the full efficacy of FM to provide HEV protection in formulations is currently under investigation, and initial gene expression studies are promising; however the remainder of this article will focus on how to formulate with the material since it imposes challenging aesthetics.
Materials and Methods
A 10% aqueous solution of FM was prepared and two batches of each test formula were made; one with and one without 1% of the FM solution. Formulation parameters were kept the same between the control and FM formula versions, including pH (6.0–7.0), preservative system, process steps and temperature of addition. Process steps were closely monitored and compared between the control and FM versions to detect any incompatibilities or other notable observations. Product samples were photographeda under controlled light conditionsb as they appeared in 2-oz. storage containers and when spread onto a surface; FM and control versions for each formulation type were photographed 24 hr after batching.
Product samples were evaluated for color, odor, appearance and texture using organoleptic methods. In addition, the viscosity of the product samples was measured using viscometersc and the pH was measured using a pH meterd. The pH of water-in-oil emulsions was measured and recorded prior to emulsification, while the pH and all other specifications of the remaining formulas were measured when freshly made and at 24 hr after batching. Samples of both the control and active versions of each formula type were placed on microscope slides and observed and photographed at 400x and 1,000x magnificatione. The morphology and size of particles and droplets were recorded. Slide samples were evaluated for possible crystallization of the FM.
Product samples in 2-oz. glass containers of the control and active formulas were then tested by an accelerated stability protocol, as described in Table 2. Photographs, microscope evaluations and specifications were recorded at predetermined time intervals. The morphology and sizes of particles and droplets were also recorded. Emulsion stability samples and microscope slide samples were evaluated for possible flocculation/aggregation, coalescence or creaming/sedimentation of particle or droplets, and possible crystallization of FM.
Results and Discussion
Microscope slide samples of the w/o emulsion sunscreen containing inorganic actives, with and without FM, showed amorphous insoluble particles under the microscope, which were suspected to be titanium dioxide sunscreen active and not FM particles or crystals. This was confirmed by recreating the formula without titanium dioxide. The new formula did not show particles when evaluated under the microscope. Readers should note that the emulsion without titanium dioxide was not stable, which was evident by non-uniform droplet morphology and size distribution.
In addition, a slurry of titanium dioxide was evaluated under a microscope and the particles found matched the morphology and characteristics of those found in the formula samples with titanium dioxide. The phenomenon of titanium dioxide agglomeration is well-known and documented, and the smaller the particle sizes, the higher the surface energy and greater the odds for agglomeration. This agglomeration leads to not only a less appealing formulation in terms of aesthetics, but also to less effective UV absorbance, as shown by Figure 3.10
FM was also found to cause an initial drop in viscosity in the gel, serum and o/w emulsion (anionic and nonionic) formulas. However, no significant changes in viscosity were observed after stability testing, compared with the control (data not shown). The pH was not affected by FM during stability testing, and FM showed impressive color stability across all conditions with no significant change in color when observed visually. Further, no changes in odor, texture or appearance of the FM samples were detected after stability testing. Evaluation of microscope slide photographs confirmed good stability of FM without agglomeration or crystallization. Overall, FM showed good compatibility and was stable after stringent stability stress conditions.
Color Mitigation Tests, Aesthetics
The addition of the FM solution was found to cause varying color changes to each formula type, from a light beige to a dark burgundy/brown color. Being a water-soluble compound, FM generated the darkest color in the toner and gel test formulations (see Figure 4). A simple pH test was thus devised to see whether pH differences would affect this color contribution of the FM. The toner and carbomer gel formulas were reproduced and their pH levels adjusted to 4.5. An additional batch for each formula was then made and the pH adjusted to 9.0. The color of each was evaluated visually and it was concluded that while formulas beyond the pH range of 5.5–6.5 containing the FM were found to darken, the color remained the same regardless of a pH of 4.5 or 9.0.
In general, formulas beyond the pH range of 5.5–6.5 containing the FM were found to darken, and heating was also found to cause darkening. The authors also observed that heating was found to cause darkening, although the addition of FM to a formulation at 45°C postemulsification maintained its stability.
In the authors’ opinions, a dark, brownish colored product may not be appealing to consumers, and after evaluation of all formula types made with FM (see Figure 5), the w/o emulsions stood out as an effective way to mitigate the color of the FM; since FM is watersoluble, this color may be concealed in the internal phase of the w/o emulsion. Two additional ideas were tested for FM color mitigation, the first including the addition of 0.1% titanium dioxide into one of the darker emulsions with FM—i.e., the o/w emulsion (anionic/ nonionic). Compared with a control of the emulsion with FM, the addition of titanium dioxide brightened the emulsion to a lighter color. For the second idea, FM was added into a color foundation formula in which its own combination of color pigments proved effective in masking FM’s color.
Summary
HEV light is an important source of skin damage that is not fully addressed with current approaches in sunscreen protection. The present work describes the creation of a compound to shield skin from HEV light, including several challenges this approach poses; for instance, the difficulty in producing a compound that will absorb at the blue wavelength range with minimal absorption in the red wavelength region that also does not penetrate the skin.
Formulating with the compound also presented challenges because in order to provide the described benefits, it must impart color. Additionally, formulations containing this compound must remain compatible with other common cosmetic ingredients and stable in cosmetic formulations. The work described herein shows how FM can be successfully incorporated into a variety of common formulation types without causing any detrimental effects to formula stability, and that its color effects were mitigated to a level deemed acceptable to the authors. Future work will include consumer preference testing for sensory acceptance.
References
Send e-mail to [email protected].
1. M Denda and S Fuziwara, Visible radiation affects epidermal permeability barrier recovery: Selective effects of red and blue light, J Invest Dermatol 128 1335–1336 (2008)
2. M Schmuth et al, Permeability barrier function of skin exposed to ionizing radiation, Arch Dermatol 137 1019–1023 (2001)
3. J Moan, Visible light and UV radiation, available at www.uio.no/studier/emner/matnat/fys/ FYS3610/h04/undervisningsmateriale/Moan7.pdf (accessed Jan 26, 2011)
4. L Zastrow, N Groth, F Klein, D Kockott, J Lademann and L Ferrero, Detection and identification of free radicals generated by UV and visible light in ex vivo human skin, IFSCC Magazine 11 3 297–315 (2008)
5. C Glazer-Hockstein and JL Dunaief, Could blue light-blocking lenses decrease the risk of agerelated macular degeneration? Retina 26 1 1–4 (2006)
6. RP Mason, KR Maples and KT Knecht, In vivo detection of free radical metabolites by spin trapping, www.rsc.org/ebooks/archive/ free/BK9780851868714/BK9780851868714- 00001.pdf (accessed Jan 17, 2011)
7. JH Lee, MR Roh and KH Lee, Effects of infrared radiation on skin photoaging and pigmentation, Yonsei Medical Journal 47 4 485–490 (2006)
8. N Dayan, V Rai and B Michniak-Kohn, Evaluation of photo-toxic effect of fractionated melanin and the relationships to cell protection activity, AAPS Annual Meeting, poster W4453 (2010)
9. Portland State University Quantum Physics document, available at http:// web.pdx.edu/~ralfw/physics/Workshop/Spring/9thQuantumPhysics.pdf pp 3, 10 (accessed Jan 26, 2011)
10. M Inkyo and T Tahara, Dispersion of agglomerated nanoparticles by microdemia mill ultra apex mill, J Soci Powder Tech Japan 41 8 1–11 (2004)