To read this article in its entirety, click through to your January 2020 digital magazine.
Vitamins have been used in cosmetic recipes for a century but the number of vitamin C, ascorbic acid and/or L-ascorbic acid-based skin care products has dramatically expanded in recent times. A regular actor in high-tech beauty routines, ascorbic acid is appearing frequently in launches including serums, oils, moisturizers, etc., that claim to help boost, improve and protect skin’s equilibrium. The reasons for this new wave of success are many.
Firstly, L-ascorbic acid is an essential molecule for humans but cells cannot synthesize it. As such, the consumption of supplements containing high concentrations of vitamin C has also increased. However, the skin is the last organ addressed by nutrients from the digestive process, so there is a concentration gap that topical vitamin C formulations could fill, helping to improve the efficiency of the skin’s metabolism.1
On the other hand, the introduction of ascorbic acid into finished formulations is difficult because this ingredient is extremely unstable.2, 3 It quickly oxidizes, especially in aerobic conditions and under exposure to light. Here, we explore the benefits of L-ascorbic and consider how to best stabilize it for delivery into skin.
History and Uses
Far from new to the industry, L-ascorbic acid and its derivatives are skin care ingredients that still create some of the most consumer buzz. To understand this continued interest, it helps to know some background on the ingredient’s use.
Vitamin C is essential for human life. Its deficiency causes scurvy; indeed, the name ascorbic is derived from the word ascorbutic, where scorbutus means scurvy. Scurvy is the result of malnourishment and typically presents with bleeding gums and the re-opening of wounds. However, in the 18th century, Lind, a British naval surgeon, found that citrus fruits rapidly cured this disease, which was common to travellers and especially seamen.4
As a supplement, the minimal dietary intake of vitamin C to prevent scurvy is 6.5 mg per day,5 which is barely enough to prevent a drop in the body’s pool of this vitamin to below 50%. Topically, ascorbic acid has important physiological effects on skin: inhibiting melanogenesis, promoting collagen biosynthesis6-8 and preventing free radical formation. These effects are all closely related to the well-known antioxidant properties of this compound.9, 10
L-Ascorbic acid also inhibits NFkB, which is responsible for the activation of different pro-inflammatory cytokines such as IL-1, IL-6 and IL-8. Therefore, ascorbic acid has potential anti-inflammatory activity and can be used to treat skin conditions such as acne vulgaris and rosacea. It also can promote wound healing and prevent post-inflammatory hyperpigmentation.11, 12
As noted, oral supplements are one way to get antioxidant vitamins into the skin but the body’s control mechanisms regulate the levels of ingested vitamins to all the various organs, so the skin only receives a tightly regulated amount. Furthermore, many individuals do not ingest an adequate supply of antioxidant nutrients through their diet. Applying antioxidants topically would there be an efficient way to increase skin levels, to help protect against reactive oxygen species (ROS) and combat cutaneous damage.
For example, in a study using porcine skin, levels of ascorbate after the topical application of a 10% aqueous solution with or without vitamin C were measured by HPLC. The resulting ascorbate content in skin was more than 25 times greater in vitamin C-treated skin than in skin treated with the vehicle alone. Although not specifically tested, these levels would be assumed to be much higher than those obtainable by oral supplementation.13
Skin Delivery
One aspect of product formulation often overlooked are the correct parameters to enable the most efficient delivery of actives through the skin. The Fick’s First Law equation indicates the diffusion/permeability of a substance through the skin as follows (see Equation 1):
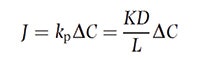 where D = the diffusion coefficient related to the molecular weight/volume and to the surface charge separation/dipole moment of the permeant; K = the partition coefficient of octanol/water; and DC is the difference in concentration between the vehicle (or starting phase) and the stratum corneum (receiving phase).14
where D = the diffusion coefficient related to the molecular weight/volume and to the surface charge separation/dipole moment of the permeant; K = the partition coefficient of octanol/water; and DC is the difference in concentration between the vehicle (or starting phase) and the stratum corneum (receiving phase).14In considering this equation, diffusion is enhanced by small molecules, i.e., the 500-Dalton rule;15 by a vehicle containing a high concentration of the permeant; and for molecules where Koct/water is as similar as possible to that of the stratum corneum.16, 17 It is important to underline that, for vitamin C, both percutaneous absorption and efficacy were evaluated and found to be proportional to its concentration but only up to 20% (see Figure 1). For unknown reasons, concentration levels higher than 20% resulted in decreased tissue levels.18, 19
In the same study, the effect of pH was also evaluated in terms of percutaneous absorption. Here, it was observed that lower the pH of the solution in which the ascorbic acid is dissolved, the higher the level of vitamin C found in the skin.
Continue reading in your January 2020 digital magazine...