As consumer interest in natural ingredients continues to grow, along with the demand for novel textures and product forms, the use of vegetable oils in personal care formulations is increasing. In fact, the consumption of natural oils in Europe is forecasted to grow at about 5% during the next five years.1 Although these natural ingredients offer distinct benefits including emolliency, gloss and lubricity,2 they also challenge formulators to provide easy application and pleasant aesthetics without a greasy or oily feel.
Lipids and silicones can act as complementary ingredients in finished formulations.3 This article illustrates how silicones such as caprylyl methicone, phenyl trimethicone, cetyl dimethicone and cyclopentasiloxane can enhance the feel of natural lipids, allowing formulators greater flexibility to expand the use of natural ingredients in their products. Even at low use levels, silicones can decrease the surface tension of vegetable oils, improve their spreading characteristics and offer a wider range of sensory profiles.
The Source for Vegetable Oils
Vegetable oils, also referred to as natural lipids, are oily substances derived from plant sources. They have a variety of chemical compositions but most used in personal care are rich in triglycerides that are mechanically extracted from the seeds of plants. The non-triglyceride components are referred to as the unsaponifiable fraction and this typically consists of tocopherols, sterols, free fatty alcohols and triterpenes.
Triglycerides are esters composed of one glycerin molecule bonded to three fatty acids—long-chain carboxylic acids in which the alkyl chain normally contains ten or more carbons. This structure is depicted in Figure 1, where R1, R2 and R3 are fatty acids.
The three fatty acids can have equal or different chain lengths and their carbon chains can be saturated or unsaturated. The fatty acid composition of triglycerides varies according to their source. For example, triglycerides derived from coconut are rich in lauric acid (saturated C12 fatty acid), and in many cases, materials such as sodium lauryl sulfate still retain a slight odor of coconut oil from which this surfactant is derived.
Based on the International Nomenclature Cosmetic Ingredient (INCI) system, natural lipids are named according to the genus and species of the plant. For example, the INCI name for borage oil is Borago officinalis seed oil.
Complementary Silicones
Silicones are synthetic polymers made from quartz, a natural form of crystalline silicon dioxide, and methanol. These materials have been used in personal care products for more than 50 years. Most silicones for personal care applications are based on polydimethylsiloxane or dimethicone. This linear polymer is available in a range of molecular weights, with the molecular weight for a particular dimethicone determining its viscosity. Volatile silicones such as cyclopentasiloxane are short-chain cyclic polydimethylsiloxanes. Another commonly used silicone is phenyl trimethicone, a highly branched phenyl-functional silicone.
Silicones are good emollients that improve the feel of formulations, while lipids act as moisturizers and can also restore the barrier function of skin.
Two vegetable oils were evaluated in this study: borage oila and a vegetable oil blendb composed of Brassica campestris (rapeseed) seed oil and Elaeis guineensis (palm) oil.
These oils were blended with four compatible silicones:
- caprylyl methicone, a caprylyl-branched liquid trisiloxane;
- phenyl trimethicone, a highly-branched, liquid phenyl-functional silicone;
- cetyl dimethicone, a linear liquid polysiloxane with alkyl chains randomly distributed, which is highly compatible with organic ingredients; and
- cyclopentasiloxane, a cyclic molecule that provides transient emolliency because of its volatility.
Lowering Surface Tension
Surface tension is a measure of the work needed to create a new surface area. High surface tension, together with high viscosity, can contribute to tackiness.4 A bubble pressure tensiometerc was used in the present study to measure dynamic and static surface tension.
Gas bubbles were produced in the sample liquids at an exactly defined bubble generation rate. As the dynamic surface tension is recorded as a function of bubble life time, the rate decreases while the bubble life time increases. The bubbles enter the liquid through a tube of known radius, calibrated before taking measurements, and the pressure reaches a maximum that is recorded by the instrument.
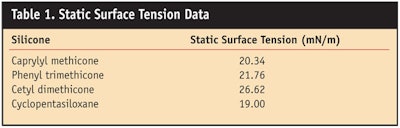
Silicones have an inherent low surface tension (see Table 1) due to the methyl groups attached to the backbone.
Figure 2 shows the decrease of dynamic surface tension of borage oil with the addition of phenyl trimethicone. Figure 3 shows the decrease of static surface tension of borage oil with the addition of cyclopentasiloxane, phenyl trimethicone, caprylyl methicone or cetyl dimethicone. Similar results were obtained with the vegetable oil blend.
Enhanced Spreading
The spreading characteristics of cosmetic oils determine how easily they can be applied and how well they will be distributed onto the skin. In general, the more readily the oil spreads, the more pleasant it will feel on the skin. A gelatin test is a common in vitro method for measuring the spreadability of cosmetic oils because gelatin is a fairly representative model of the human skin surface. To conduct this test, a gelatin film is applied to polystyrene plates (Petri dishes) and a 5-µL sample of the cosmetic oil is applied onto the film. A stereomicroscope is used to measure the diameter of the oil droplet at time zero and after 10 min. Equation 1 shows how spreadability is calculated.
Eq. 1
Values can be compared only when they have been determined under identical humidity and temperature conditions, and the average of at least three measurements should be used. Results are expressed as an enhanced spreadability factor (see Equation 2).
Eq. 2
This method was used to determine the effect of silicone on the spreadability of vegetable oils. If the enhanced spreadability factor is found to be greater than 1, the additive improves spreadability; if it is less than 1, the additive decreases spreadability.
Figure 4 shows how the spreadability and surface tension of borage oil can be influenced by the addition of silicone. The improvement of spreadability depends upon the type and level of silicone used.
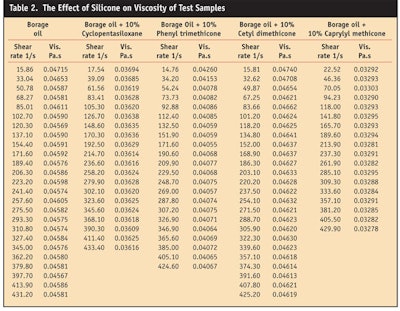
The effect of silicone on viscosity also was studied using a rheometer and a stress ramp procedure (see Table 2 and Figure 5).
Results demonstrated that borage oil and its blends with silicone are Newtonian liquids. The addition of cyclopentasiloxane, phenyl trimethicone or caprylyl methicone decreases the viscosity of borage oil, while the addition of cetyl dimethicone increases its viscosity slightly. The greatest reduction in viscosity was obtained with caprylyl dimethicone.
Additional trials were carried out with the vegetable oil blend (Figure 6).
These results show that spreadability improvement can be achieved for more than one type of vegetable oil with the addition of silicone.
Sensory Attributes
Generally formulators are most interested in sensory enhancements that can be perceived on the skin; thus, a series of sensory panel tests was also conducted. Comparisons of pure oils and the same oils blended with silicone were tested by an experienced sensory panel of 18 Caucasian participants. The sensory evaluations were performed in a controlled climate, with humidity at 50% ± 5%, and the temperature at 20°C ± 2°C. Each panelist applied 0.02g of both product samples and assigned scores for several sensory attributes during rub-in as well as after they perceived the product had been absorbed on the skin—i.e., the ratings were based on panelists perceptions, not biological skin absorption. For example, panelists found that 5% cetyl dimethicone improved a number of sensory attributes of a vegetable oil blend (see Figures 7 and Figure 8). They noted less greasiness and a lighter skin feel during rub-in and after absorption, and less gloss after absorption.
Other sensory evaluations revealed that:
• 20% caprylyl dimethicone gave a lighter skin feel for borage oil during rub-in;
• 10% phenyl trimethicone reduced the tackiness of borage oil during rub-in;
• 10% cyclopentasiloxane improved the skin feel of borage oil, resulting in less greasiness during rub-in and a lighter skin feel during rub-in and after absorption; and
• 10% cyclopentasiloxane improved the feel of the vegetable oil blend, making it easier to spread and less tacky during rub-in.
Prototype Formula 1, Formula 2 and Formula 3 illustrate the use of silicones with the vegetable oil blend.
Discussion
The addition of caprylyl dimethicone resulted in a significant decrease in the surface tension and viscosity of vegetable oils and that can be translated as enhancement of spreadability on gelatin. Panelists confirmed the improved sensory properties obtained for borage oil with 20% caprylyl dimethicone. Lower addition levels were not tested by the sensory panel.
Phenyl trimethicone also decreased the surface tension and viscosity of vegetable oils. The addition of 20% phenyl trimethicone resulted in a significant improvement of the spreadability of borage oil. The panel test showed improvement of the skin feel of borage oil with the addition of 10% phenyl trimethicone.
Cetyl dimethicone slightly decreased the surface tension of vegetable oils and slightly increased their viscosity; however, results showed an improvement in spreadability and sensory properties. Panelists were able to detect a sensory difference at 5% cetyl dimethicone in the vegetable oil blend.
The greatest reduction in surface tension was found with cyclopentasiloxane. Panelists confirmed that the addition of 10% cyclopentasiloxane in borage oil or in a vegetable oil blend improved sensory attributes.
Summary
The present study shows that it is possible to lower the surface tension of vegetable oil and improve its spreadability with the addition of silicones such as caprylyl dimethicone, phenyl trimethicone, cetyl dimethicone or cyclopentasiloxane. In some cases, the improvements were confirmed by sensory panel testing.
Combining vegetable oils and silicone is one way for formulators to obtain improved sensory characteristics and broaden the opportunity to create innovative skin care products that expand the use of natural materials.
References
1. Kline market report, Specialty Raw Materials for Cosmetics and Toiletries Volume III: Western Europe (2006)
2. M. Rieger, Cosmetic use of selected natural fats and oils, Cosm & Toil 109 57–68 (1994)
3. AL Girboux and M Starch, Formulation with silicone and natural lipids, Happi 42(12) 100–104 (Dec 2005)
4. U Zeidler, Über die taktilen eigenschaften kosmetischer öle, Upon tactile properties of cosmetic oils, SÖFW, 118 1001–1007 (1992)