
In 2011, O’Lenick et al. wrote that the temperature at which the volatility of a silicone is measured is key to selecting a candidate for replacing D5.
“Volatility is the ability of the compound being tested to evaporate under the temperatures at which the compound is used in formulation. For cosmetic products, this temperature is ambient (~25°C). It has generally been accepted that cyclomethicone compounds provide this feel because they evaporate quickly after helping to carry oils to the top layer of the epidermis. The ability to provide a product that (1) has the dry feel, (2) is cyclomethicone and (3) is not flammable is a long-felt, unsatisfied need in the cosmetic industry.”
They added, “Personal care products are unique in that they are not applied at elevated temperatures, as are many industrial products. It is therefore unreasonable to talk about the volatility of a cosmetic solvent at elevated temperatures. The temperature for industrial volatility testing is typically 200°C, which is clearly not useful to personal care evaluations.”
Silicone Materials at 20°C
The data published in 20111 showed that under the conditions studied (20°C), 86.1% D5 liquid remained after 24 hr, indicating it does not easily evaporate. Table 1 and Figure 1 show these results, which were obtained by placing, in a 250-mL beaker, 100 g of silicone, which was maintained at the specified temperature.
Table 1. Percent by weight of silicone remaining @20°C1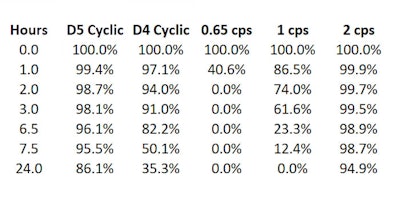
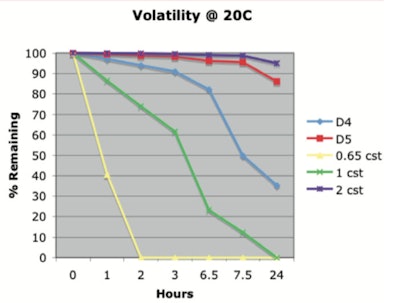
The rate of evaporation was measured by adding 3.00 g of each sample to an aluminum dish. Each aluminum dish had a 7-cm diameter and was placed in a 37°C oven and weighed every 10 min. Table 2 and Figure 2 show the results.
Figure 2. Percent by weight of silicone remaining 37°C (thin film)
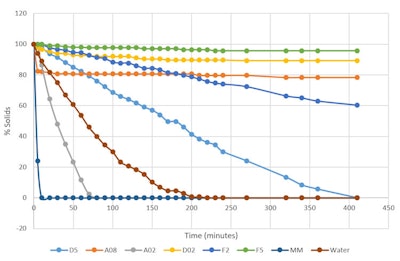
Table 2. Percent by weight of silicone remaining 37°C (thin film)
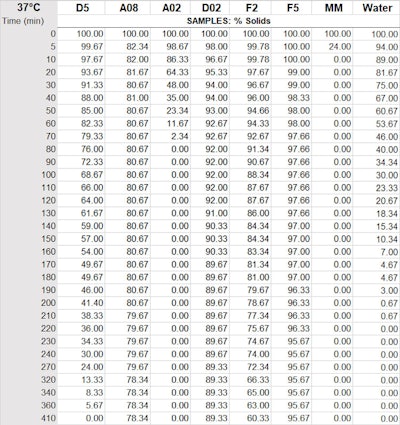
Legend: D5 = cyclopentasiloxane; A02 = ethyl trisiloxane; D02 = ethyl dimethicone; A08 = octyl trisiloxane; MM = 0.65 visc. fluid (disiloxane); F2 = 2 visc. fluid; F5 = 5 visc. fluid
Discussion
Figure 2 as noted includes several new silicone polymers; it also points out that under this type of test methodology, D5 evaporates in 400 min—taking twice as long as water. From fastest to slowest evaporation time (to 0 g), the volatile samples were: MM 0.65 visc. fluid (disiloxane) < A02 ethyl trisiloxane < water < D5 cyclopentasiloxane. The non-volatile samples, ranked in the same manner, were: D02 ethyl dimethicone < A08 octyl trisolixane < F2 2 visc. fluid < F5 5 visc. fluid.
Conclusions
The proposed changes provided a good screening methodology. Therefore, it is suggested that the evaporation rate of the final formulation be evaluated using this revised methodology. The conclusion drawn in 2011 also remains accurate, illustrated as follows.
Question: The assumption that volatility is required for a dry feel is due to the fact that historically, D4 has a dry feel and is volatile. D5 has replaced D4 in formulations and consequently, has been deemed acceptable in many cosmetic formulations. But is volatility what is primarily responsible for the dry feel?
Answer: Since the cosmetic industry has accepted D5 as a replacement for D4, and it clearly is much less volatile at temperatures the consumer is likely to encounter, the dry feel must be caused by other factors.
The above information provides a good testing methodology; however the formulator must ask: Why is cyclomethicone used in formulations? The answer must always be functional, not chemical. It was chosen because it gives the dry feel that is so desirable in many formulations.
The chemistry present in the raw material that provides this benefit is then evaluated, but it is a property in formulation, not in chemistry, that must be reproduced in a viable replacement. In conclusion, the salient property that any potential replacement would need is the silky smooth feel on skin. The next is cost, followed by the lack of flammability.2










