The June 2008 issue of Cosmetics & Toiletries magazine featured an article from Cognis on the mildness of alkyl polyglucoside (APG) surfactants. The article stimulated the following comment from a reader: “Sugar-based ingredients are better sources of nutrients than fatty-based ingredients. Doesn’t a surfactant made of water and sugar present an especially attractive growth medium for microorganisms? Does that mean APGs require special preservation measures in formulations, and if so how would that challenge be met in an Ecocert setting?”
Formulations in which APGs are incorporated do not require special preservatives beyond what already are required by the rest of the ingredients, according to Barry Salka, who served as technical manager at Cognis for 23 years and currently is performing similar duties at Surfactants Inc.
“A shampoo without APGs and a shampoo with APGs would need the same kind of preservation,” Salka told C&T magazine.
Ken Klein, president of Cosmetech, agrees. “Glucosides are no more difficult to preserve than other surfactants that are basically 30% solutions in water,” Klein said. How are APGs preserved? Some answers to this question are surveyed here.
Alkyl Polyglucosides
To begin with, a glucoside is a glycoside that is derived from glucose. In a glycoside molecule, the sugar part is bound to some other part. Many plants store important chemicals in the form of inactive glycosides; if these chemicals are needed, the glycosides are brought in contact with water and an enzyme and the sugar part is then broken off, making the chemical available for use.2
In 1893 German chemist Emil Fischer combined fatty alcohols and glucose obtained from coconut or palm kernel oil and corn for the first time, creating the first alkyl glucosides. Nearly a century passed before an industrial process was developed to manufacture these glucosides. But whereas Fischer used hydrophilic alcohols such as methanol and glycerol, resulting in pure alkyl monoglucosides, the industrial process uses hydrophobic alcohols with alkyl chains from octyl (C8) up to hexadecyl (C16) glucosides, resulting in a complex mixture of alkyl mono-, di- tri- and oligopolyglucosides. For this reason, the alkyl glucosides produced in the industrial process are widely known as alkyl polyglucosides.3
In alkyl polyglycosides, the sugar is corn starch glucose and the “other part” is a fatty alcohol. The chemistry (Figure 1)
and typical structure (Figure 2) of an alkyl polyglycoside surfactant were described by Salka in C&T magazine in 1993.4 He noted that its solubility can be attributed to the many hydroxyl groups present, rather than to ethylene oxide, as is the case in typical nonionic surfactants. He also cited the work of other researchers who established that the polyglycosides have a much greater tolerance for electrolytes than conventional, ethylene oxide-based nonionics. They can effectively lower both surface and interfacial tension. They offer good detergency and a high degree of biodegradability.
Again, glucosides are glycosides derived from glucose, which is a monosaccharide or simple sugar. The simplest glucosides are the alkyl ethers that have been obtained by reacting hydrochloric acid on alcoholic glucose solutions.5 A better method of preparation is to dissolve solid anhydrous glucose in methanol containing hydrochloric acid.
One example of an alkyl polyglycoside that is also an alkyl polyglucoside (APG) is decyl glucoside (see Figure 3),
a plant-derived glucoside used as a mild nonionic surfactant in baby shampoo and in products for individuals with sensitive skin.6 Decyl glucoside is produced by the reaction of glucose from corn starch with the fatty alcohol decanol, which is derived from coconut. It has a broad alkyl chain length range—C8 to C16—but the chain length averages 10 carbons.
“The choice of alcohol used to prepare the alkyl polyglucoside has a dramatic effect on its properties,” wrote Tony O’Lenick, currently of Siltech Inc., in 1999. “The lauryl range products (12 carbons) act as thickeners in anionic systems and build foam stability. In this regard, they act like alkanolamides. The decyl range products (10 carbons) provide detergency, foam and surfactant properties as the primary surfactant. Alkyl polyglucosides will almost certainly grow in significance in the personal care area as the various manufacturers introduce more custom-tailored products.”7
Preserving Alkylpolyglucosides
If, as Salka and Klein say, preserving formulations that contain APGs is no different than preserving formulations that do not contain APGs, then what approach is the industry using to preserve cosmetic formulations? Experts have written books on that subject, three8–10 of which are cited in the following three paragraphs.
Karl H. Wallhäuser monographed more than 120 antimicrobial preservatives used by the cosmetic industry in 1984.8 He also listed them in a table showing, for each preservative, the use concentration, optimal pH range, frequency of use in US cosmetics, and spectrum of activity against bacteria, molds, and fungi and yeasts.
David C. Steinberg monographed more than 60 traditional preservatives and their commercial mixtures in 2006.9 He also discussed multifunctional ingredients—actives, antioxidants and chelating agents—that also have antimicrobial activity. The regulatory status of these preservatives is listed in tables showing the maximum concentration allowed and other limitations and warnings that apply in the United States, Brazil, Canada, the European Union, Japan and Mexico. “The ideal preservative does not exist and probably cannot exist,” Steinberg writes, as he considers all the desired properties the ideal preservative should have and the regulatory and safety challenges it must meet.
At least one book has been dedicated to preservative-free and self-preserving cosmetics.10 It discusses hurdle technology and the antimicrobial action of essential oils, surfactants, fatty acids and fatty acid esters, as well as chelating agents and antioxidants. The authors emphasize that preservation of a product should be inherent in the formula itself.
The pH of the finished formulation is critical to the success of the preservative because some preservatives, such as organic acids, are solids that are soluble in water (where the microbes are) only when the formulation pH is within certain ranges. A high pH is used by some suppliers to preserve APGs in bulk. For example, Cognis’ personal care alkyl polyglucosidesa are supplied at a pH of 11.5–12.5 with no preservative. “Alkalinity keeps bugs from growing,” Salka said. Extreme acidity, such as pH around 2, can have the same effect.
Here are a few examples of the commercial preservatives used by various manufacturers to preserve formulations containing decyl glucoside.
• A mixture of diazolidinyl urea, methylparaben, propylene glycol and propylparabenb in a 2002 L’Oréal patent on hydrous salicylic acid solutions;
• Diazolidinyl urea and iodopropynyl butylcarbamate in several LanaB foaming products;
• Broad spectrum preservative blends that incorporate caprylyl glycol with either chloroxylenol or chlor-phenesin or both in a 2006 patent application11 from Arch Chemicals.
The mixturec of methylchloroisothiazolinone and methylisothiazolinone is a long-time favorite preservative. One example with this preservative in an APG-containing formulation is a low irritation bath gel containing lauryl glucosided cited by Salka in a 1993 article in C&T magazine.12
Preserving Alkyl Polyglucosides Under Ecocert
Synthetic preservatives, like those mentioned above, could never be used in a formulation claiming conformance to Ecocert. Ecocert is an organic certification organization, founded in France in 1991. It is based in Europe but conducts inspections in more than 80 countries, making it one of the largest organic certification organizations in the world.
“Consumers in Europe don’t want any more parabens and phenoxyethanol or other preservatives in their products,” said Corrine Garnier, a spokesperson for Ecocert. “So they read the INCI list on those products.”13
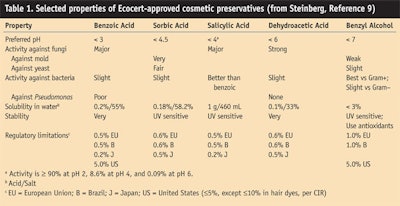
The list of preservatives approved by Ecocert for cosmetics contains only five names: benzyl alcohol, salicylic acid, benzoic acid, sorbic acid and dehydroacetic acid, listed roughly in order of their effectiveness up to increasingly higher pH. For example, the optimal pH range is up 4 for benzyl alcohol and 7 for dehydroacetic acid, according to Wallhäuser.14 “Generally manufacturers use a mixture of two or three of these preservatives to be more efficient,” Garnier said.
Table 1 presents selected data from Steinberg’s monographs of the Ecocert preservatives. It shows the numerous factors that can affect a formulator’s choice in selecting one or more of these preservatives for a formulation with or without APGs. It is not an easy task. As Ken Klein remarked, “The preservatives listed in Table 1 would work fine, except the problem would be they require a very low pH because they must be in the acid form in order to work. So the pH should be below, say, 4. When the pH is 4 or lower, a number of materials found in typical shampoo formulations would tend to hydrolize or break apart. Therefore, I’m not so sure how effective they would be at the pH normally used.”
Here’s more on the critical role of formulation pH from Paul Ching, technical manager for Seppic Inc. in Fairfield, N.J. Seppic makes a commercially available decyl glucosidee and a seriesf of emulsifiers based on various glucosides. Both products are 100% natural in origin and validated for use in Ecocert-certified products.
Seppic also offers several ingredients that can be used to preserve a lotion or cream. The company makes two ingredients called undecylenoyl glycine and capryloyl glycine. It is also the sole distributor for another ingredient called glyceryl undecylenate. All three are Ecocert approved, but none of the three is listed or certified as a preservative by Ecocert.
“Though they aren’t listed as a preservative, you can use them and they kill bacteria, mold and fungus,” Ching said. “They are acids by nature but in order for them to be effective, you have to work with the specific pH of each individual product because alone, each of these ingredients is a solid, and you have to utilize it at a certain pH before it can be soluble in water.
“For example, the benzyl alcohol ceases being active when the formulation pH goes above 7. The benzyl alcohol is still in the formulation, but it is in a different form, so it will not have the ability to kill whatever you wanted it to kill. The pH is very important. Each one of them is different. It is best to talk to the supplier before using them.”
The supplier plays a critical role in developing formulations to be marketed with a preservative-free claim, according to Ching. “If your product contains an ingredient that is not on a preservative list but the supplier has run challenge tests demonstrating that the ingredient has the ability to kill mold or fungus, then that ingredient serves the function of a preservative without being labeled as one,” Ching said. “Manufacturers use those ingredients because they are vegetable-based and can preserve a lotion or cream intended for marketing with a preservative-free claim.”
Certainly the Natura Organics line of vegan products would satisfy Ecocert. The Soothing Shower Gel contains decyl glucoside and two Ecocert-listed preservatives: potassium sorbate and sodium benzoate.
Pure Earth Foaming Facial Cleanser lists olive leaf extract and hydro glycinate as “p (Figure 1) lant based preservatives” in an ingredient list that also includes decyl glucoside.
For a final example, consider a surfactant based on alkyl polyglucoside quaternary compounds described in a 2005 Colonial Chemical patent.15 The surfactant provides detergency, foam, conditioning, antimicrobial activity and wetting properties in a single molecule derived from natural sugar compounds.
The inventors speculate on the possibility of making shampoos and body wash products with only one surfactant. Perhaps the surfactant would have enough antimicrobial activity to make the formulation self-preserving.
“What our invention teaches,” the inventors write, “is that less is better—if that ‘less’ comes from one unique natural surfactant that provides all the needed benefits in a single molecule.”
Comment
Preservatives have always faced a safety issue: chemicals that kill microbes can sometimes also kill mammalian cells. But today preservatives also face a sourcing issue: consumers want the option for products whose ingredients, including preservatives, are plant-based. Some consumers will go a step further and demand those plants be certified as having been grown under organic conditions. The job of preserving a formulation—whether it contains alkyl polyglucosides or not—is harder today than it was yesterday.
References
1. A Mehling, G Pellón and H Hensen, Greenness meets mildness, Cosmet Toil 123(6) 53–58 (2008)
2. http://en.wikipedia.org/wiki/Glycoside
3. K Hill, W von Rybinski and G Stoll, Alkyl Polyglycosides: Technology, Properties and Applications, K Hill, W von Rybinski and G Stoll, eds, New York: Wiley (1997) p 1
4. B Salka, Alkyl polyglycosides: Properties and applications, Cosmet Toil 108(3) 89 (1993)
5. http://en.wikipedia.org/wiki/Glucoside
6. http://en.wikipedia.org/wiki/Decyl_glucoside
7. AJ O’Lenick Jr, Surfactants: Chemistry and Properties, Allured Publishing: Carol Stream, IL 88 (1999)
8. KH Wallhäuser, Antimicrobial preservatives, in Cosmetic and Drug Preservation: Principles and Practice, JJ Kabara, ed, Marcel Dekker, New York 605–745 (1984)
9. D Steinberg, Preservatives for Cosmetics, Allured Publishing: Carol Stream, IL (2006)
10. Preservative-free and Self-preserving Cosmetics and Drugs: Principles and Practice, JJ Kabara and DS Orth, eds, Marcel Dekker: New York (1997)
11. WO/2006/033970, Broad spectrum preservation blends, DT Ciccognani, KN Dinicola, SD Hinden and KP Roberts, applied for by Arch Chemicals (Mar 30, 2006)
12. Ibid ref 4, p 94
13. C Garnier, Personal communication dated May 19, 2008
14. Ibid ref 8, p 615–618
15. US Pat 6881710, Personal care products based upon alkyl polyglucoside quaternary compounds, AJ O’Lenick Jr, DA Smith and D Anderson, assigned to Colonial Chemical Inc. (Apr 19, 2005)