Straight chain 1,2-alkanediols have been used for more than 15 years as moisturizers with multifunctional activities.1–3 In fact, pentylene glycol, also known as 1,2-pentanediol, was the first of the 1,2-alkanediols introduced to the cosmetic industry in the early 1990s. Since then, 1,2-hexanediol (see Figure 1a); caprylyl glycol also known as 1,2-octanediol (see Figure 1b); and more recently, decylene glycol or 1,2-decanediol, also have become available in the personal care industry.
Short chain 1,2-alkanediols are amphiphilic compounds and thus, like 1,2-pentanediol and 1,2-hexanediol, are soluble both in water and cosmetic esters. On the other hand, 1,2-decanediol is a solid and soluble only in cosmetic esters. Apart from moisturizing and anti-microbial activities, some 1,2-alkanediols are used as solvents for various cosmetic actives and viscosity modifiers.
The present research reports the results of studies on the anti-microbial properties of straight chain 1,2-alkanediols, mixtures of them, and combinations of 1,2-alkanediols with other preservatives and antimicrobial compounds. Here, the authors evaluate the potential of 1,2-alkanediols for use as preservative boosters to reduce the levels of preservatives required in formulations. In addition, the authors discuss formulating with 1,2-alkanediols to optimize formulations for stability and efficacy.
Material and Methods
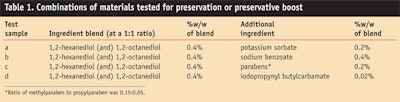
The materials tested were either synthesized in the authors’ labs or purchased from suppliers (see Table 1).
Minimum inhibitory concentrations (MICs) of 1,2-alkanediols against selected microorganisms were determined by standard agar dilution method.4 With this method, petri dishes are charged with freshly prepared agar solution to which various concentrations of test samples with potential antimicrobial activity are added. After solidification and drying, the test dishes comprising test compounds at different concentrations are inoculated with 1μL of the respective test microorganism suspensions. Inoculated plates are incubated under varying conditions depending on the type of the test organism. The MIC is the lowest concentration of active compound at which no growth of microorganism is observed macroscopically.
To evaluate preservative efficacy, the authors used a standard challenge test according to the European Pharmacoepeia.5 Cosmetic emulsions were separately inoculated with a single culture of standard microorganisms, typically in the range of several 100,000 cfu/mL (cfu = colony forming units). The microorganisms used included the Gram-positive bacterium Staphylococcus aureus; Gram-negative bacteria Escherichia coli and Pseudomonas aeruginosa; yeast Candida albicans; and mold Aspergillus niger. The inoculated emulsions were then stored over a period of 28 days and changes in the germ counts were measured at days 2, 7, 14 and 28.
The preservative system is considered to be effective if a 3 log reduction in germ count can be achieved for bacteria after two days and if a 3 log reduction for yeast and mold can be achieved after seven days. In addition to complete kill of bacteria, complete kill of yeast and mold should be achieved within 28 days.
A simple, nonfragranced anionic emulsion was formulated as the base for these tests (see Formula 1).
For the studies to evaluate the impact of 1,2-alkanediols on the stability of formulations, an anionic oil-in-water emulsion was used (see Formula 2). Here, the authors evaluated the influence of two parameters on the viscositya of the emulsion: the addition of 1% w/w each of 1,2-hexanediol, 1,2-octanediol or a 1:1 mixture of the twob; and the order of addition of these compounds—i.e., in water phase or post-emulsification stage.
Results and Discussion
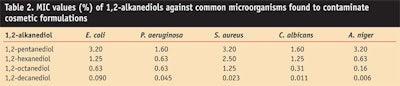
Minimum inhibitory concentrations: The MIC of straight chain 1,2-alkanediols containing 5–10 carbon atoms was measured against microbes that are common to cosmetic preservation (see Table 2).
Test results showed that the longest chain alkanediol, 1,2-decanediol, had lower MIC values and hence a higher antimicrobial activity than the shorter analogues. However, the first preservative challenge tests with 1,2-decanediol showed that it is quite ineffective (data not shown). For this reason, further studies were focused on 1,2-hexanediol, 1,2-octanediol and mixtures thereof.
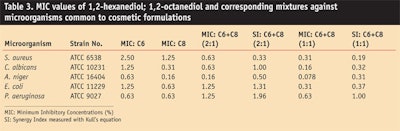
Additional MIC assessments for 1,2-hexanediol, 1,2-octanediol and their mixtures showed that, in most cases, the mixtures possessed lower MIC values than single alkanediols alone. Higher antimicrobial activity was observed especially for binary mixtures of 1,2-hexanediol and 1,2-octanediol, which showed a synergistic activity (see Table 3).
Calculations of synergism indices (SI) derived from Kull’s equation6, 7 support the notion that a synergistic activity occurs within the specific 1,2-alkanediol combinations. The Kull’s equation for measuring synergy index SI of binary mixture of X and Y is:
Eq. 1
SI = Am x Cx / Ax + Am x Cy / Ay
where Cx and Cy are the concentrations of X and Y in the mixture, and Ax, Ay and Am are the activity of X, Y and the mixture. Calculation of the SI of the binary mixture of 1,2-hexanediol and 1,2-octanediol is shown by the following example, based on the MIC against
S. aureus:
Ax = MIC of 1,2-hexanediol or 2.50;
Ay = MIC of 1,2-octanediol or 1.25;
Am = MIC of mixture of 1,2-hexanediol + 1,2-octanediol or 0.31;
Cx = Content of 1,2-hexandiol in mixture or 0.5; and
Cy = Content of 1,2-octanediol in mixture or 0.5.
Using Kull’s equation, the calculation therefore is:
SI = 0.31 x 0.5/2.50 + 0.31 x 0.5/1.25 = 0.19
In Kull’s equation, an SI = 1 designates there is not a synergistic effect; an SI > 1 indicates an antagonistic effect; and an SI <1 is evidence of a synergistic effect. Through such calculations, except in the case of P. aeruginosa where SI = 1, the synergy indices for 1,2-hexanediol and 1,2-octanediol combinations at a ratio of 2:1 and 1:1 were <1, indicating a synergistic effect (see Table 3).
Challenge tests on 1:1 blend of 1,2-alkanediols: Challenge test results on an anionic emulsion base containing 0.5% or 1.0% w/w of a 1:1 blend of 1,2-hexanediol and 1,2-octanediol are shown in Figure 2. Here, the alkanediol blend was found to have excellent activity against bacteria but weaker activity against yeast and mold, especially A. niger.
Challenge tests on 1:1 blend of 1,2-alkanediols and preservatives: Since the criteria for materials to pass preservative challenge tests often require a faster kill rate for mold than what the first test results indicated, additional studies were conducted to adjust formulations for broad-spectrum activity. Thus, the authors combined 1,2-alkanediol blends with various doses of preservatives such as sodium benzoate, potassium sorbate, a paraben blend (methylparaben and propylparaben, 3:1 ratio) and iodopropynyl butylcarbamate. The results of the challenge tests on these combinations showed that combining lower doses of preservatives than are typically used with the 1,2-alkanediol blend produced a robust preservative system for topical formulations (see Figure 3).
Besides the aforementioned compounds, the authors noted excellent efficacy was observed by combining 1,2-alkanediol blends with dehydroacetic acid, benzyl alcohol and phenoxyethanol (results not shown).
Screening for other antimicrobial compounds: Since the 1,2-alkanediols did not provide a 3 log reduction of the mold count after seven days of incubation, the authors initiated a program to screen for other antimicrobial compounds that could be combined with alkanediols to preserve formulations. During these studies, different product types commonly used in fragrance and cosmetic products were evaluated—including chelating agents, deodorant actives, antioxidants and aroma chemicals, alone and in combination with 1,2-alkanediols.
The tested materials include benzo-phenone-1, benzophenone-2, bisabolol, climbazole, dimethyl phenylpropanol, disodium EDTA, glyceryl laurate, ethylhexylglycerine, hinokitiol and polyglyceryl-3 caprylate.
Yeast and mold proved to be challenging once again, as the test results showed none of the materials tested, alone or in combination with 1,2-alkanediols, to possess strong activity. Some activity was noted in individual and combined test samples but most materials did not show a significant reduction of A. niger within a time period of seven days. This is the general requirement indicating adequate preservation.
However, the authors noted that two compounds—2-hydroxy-2,4,6-cycloheptatriene (INCI: tropolone, see Figure 4a) and p-tolyl alcohol (proposed INCI: cresyl alcohol, Figure 4b)—showed high activity in the screening assays, especially when combined with 1,2-alkanediol at a lower concentration.
Tropolone is a chelating agent and an excellent antioxidant that is used in the pharmaceutical industry.
Cresyl alcohol is a fragrance component and deodorant.
Challenge tests combining 1,2-alkanediol blend and other antimicrobials: Preservative challenge tests conducted in the sample anionic emulsion base indicated that the 1,2-hexanediol/1,2-octanediol mixture enhanced the antimicrobial activity of tropolone and cresyl alcohol against all microbes, including yeast and mold (see Figure 5).
The authors observed a complete kill of even A. niger within one week. Other compounds that showed good preservative activity in combination with 1,2-alkanediols included disodium EDTA, bisabolol and dimethyl phenylpropanol (data not shown).
Evaluating the influence of 1,2-alkanediol on the stability of emulsions: 1,2-Alkanediols are known to affect the stability of some emulsions by lowering their viscosity. The influence of 1,2-hexanediol, 1,2-octanediol and their 1:1 mixture on the viscosity of an anionic oil-in-water emulsion was evaluated by measuring the viscosity of emulsions containing each of them (see Table 4). The alkanediols were added in water phase or at the post-emulsification stage to also determine whether the order of addition makes a difference in the viscosity of the formulations.
The results confirmed that 1,2-alkanediols affect the viscosity of emulsions adversely. 1,2-Octanediol lowered the viscosity of emulsions more than 1,2-hexanediol and the 1:1 mixture of 1,2-hexanediol and 1,2-octanediol (see Table 4). The authors also found that adding the 1,2-alkanediol mixture to the formulation after the emulsion was formed did not lower the viscosity as much as adding it in the water phase.
Discussion
1,2-Alkanediols are multifunctional compounds since they are moisturizers1 with antimicrobial activity. A recent study that evaluated the mechanism of antimicrobial activity of straight chain 1,2-alkanediols has shown that their mode of action is through the disruption of the microbial membrane.9 Though their antimicrobial activity increases with increasing chain length of the molecule, their preservative activity does not follow this order. For example, 1,2-decanediol has poorer preservative efficacy than its lower chain analogues, although its antimicrobial activity is higher.8 It could be speculated that this is due to the low water-solubility of the longer chain alkanediols. In addition, a blend of 1,2-hexanediol and 1,2-octanediol was found to provide higher antimicrobial activity against the microbes relevant to product contamination than individual 1,2-alkanediols.
The present studies illustrate how binary mixtures comprising 1,2-alkanediols can be effectively used with small concentrations of preservatives to preserve topical cosmetic formulations. This 1,2-alkanediol mixture also could be used in combination with chelating agents like tropolone and disodium EDTA and antioxidants, which are known to assist in preserving formulations.9
Another interesting finding of the studies was the impact of the 1,2-alkanediols on the stability of emulsions. Though all the tested alkanediols reduced the viscosity of emulsions to varying degrees, it was observed that the impact is higher with longer chain analogues. The authors attribute this property to their high surface activity compared with the lower analogues. In the case of the 1:1 mixture of 1,2-hexanediol and 1,2-octanediol, the viscosity reduction could be minimized by adding it at the post-emulsification stage. This bodes well for the antimicrobial efficacy also since the preservative efficacy of 1,2-alkanediols in emulsions has been shown to have higher efficacy when added to formulations after the emulsification stage.10
Conclusion
A binary mixture of 1,2-hexanediol and 1,2-octanediol (1:1 ratio) has been shown here to exhibit synergistic antimicrobial properties and, in combination with preservatives, can offer cosmetic formulators a tool to reduce the levels of preservatives used in formulations. This alkanediol mixture also can be used in combination with certain compounds such as disodium EDTA, tropolone and cresyl alcohol to effectively preserve topical formulations. Though 1,2-alkanediols can affect the stability of some emulsions by reducing their viscosity, here the authors offer formulating tips to chemists to reduce this effect.
References
1. Patent EP0655904 Use of alkane diols in cosmetics, Dragoco Gerberding Co. AG (2002)
2. Patent EP1269983 Use of 1,2-decanediol against germs which cause body malodour, Gerberding Co. AG (2003)
3. R Pillai, G Schmaus, S Lange and J Röding, 1,2-pentanediol: A multifunctional ingredient for personal care applications, SÖFW 131(6): 13–22 (2000)
4. Deutsches Institut für Normung, DIN 58940/ICS and DIN 58944/ICS
5. European Pharmacopoeia 4th Edition (2002)
6. FC Kull et al., Mixtures of quarternary ammonium compounds and long-chain fatty acíds as antifungal agents, Applied Microbiology 9, 538–541 (1961)
7. DC Steinberg, Measuring synergy, Cosmetics & Toiletries 115 (11) 59 (2000)
8. G Schmaus, R Pillai, S Lange , H Joppe and J Röding, Aliphatic 1,2-diol combinations: A safe way to reduce preservatives in cosmetic formulations; proceedings of the 23rd IFSCC International Congress, Florida (2004)
9. Preservative-free and self-preserving cosmetic and drug products: Principles and practices, J Kabara and D Orth, eds, Cosmetic Science and Technology series 16, ISBN 0-8247-9366-8 (1997)
10. S Schnittger, G Schmaus, R Pillai and J Röding, Use of 1,2-Alkanediols in personal care formulations—closer look at antimicrobial activity, in proceedings of the SCC Annual Scientific Meeting & Technology Showcase, New York (2006)