Nature demonstrates various protective strategies in the animal and plant kingdom. At the macroscopic scale, mobility (i.e., the ability to run) is the first and most obvious example, but slow or immobile creatures have evolved other fascinating ways to protect themselves. Chemical strategies can play an important role in this context. Strong odors and bright colors are often employed to point out that an attack may bring more harm to the aggressor than to the prey. This strategy depends on the large variety of naturally toxic materials produced by animals and plants in their “natural laboratories” in which some of the world’s most effective poisons are developed. Figure 1 gives the structures of some exemplary natural poisons.
Somewhat more hidden and therefore less known are the activities in the microscopic world. The strategy for chemical defense against microorganisms will normally differ from that against larger invaders due, in part, to differences in the metabolism. While large alkaloids or polypeptides may successfully harm animals, it is sometimes small and simple molecules that are effective against bacteria or fungi. Chemical defense against microbes is normally found in plants or other competing microorganisms, while animals rely on their biological immune response.
The following discussion will focus solely on the chemical defenses against microorganisms. It will define some general principles that can be exploited to determine metabolic weaknesses for the control of target organisms. It also will point out how natural systems protect themselvesi.e., which chemical compounds are best against the permanent pressures of harmful microbes. Finally, it will demonstrate the concepts that currently are available to the industry to achieve natural and sustainable—yet efficient—preservation of cosmetic products.
Throughout this discussion, the term natural is used to describe preservatives and will have a meaning as clarified by the relevant European certification bodies—BDIH (the Federation of German Industries and Trading), EcoCert (France) and the Organic Soil Association (UK) (see Petrochemical Preservatives and the Term Natural).
Natural Strategies Against Microorganisms
With the discovery of penicillin in 1928, the Scottish biologist Alexander Fleming identified one of the most prominent examples of a potent biologically active agent. This historic event has served as the foundation from which to consider different concepts in the control of microorganisms. Penicillin is an antibiotic agent and must be distinguished from active compounds for use in preservation or disinfection. The major difference is the selectivity against the target organism and as a result thereof, the possibility for the bacteria to build up a resistance. Because of their selectivity, antibiotics can be used within the human body to fight bacteria.
Active compounds for preservation are not selective, nor should they be.
Ideally their mission is to fight all microbes present—bacteria, yeasts and fungi.
The desired antimicrobial effect is directed by some general principles:
• denaturization of membrane proteins and enzymes (alcohols);
• chemical alteration of enzymes and DNA (aldehydes);
• oxidation of membrane proteins and enzymes (ozone, H2O2, halogen compounds);
• disturbance of membrane function (organic acids); and
• disruption of the cell membrane (surfactants, alcohols).
Plants, animals and microorganisms employ these principles and produce the respective compounds to combat microbes. Often, mixtures or synergistic systems are in place to fight the imminent invasion.
Phytoalexins are antimicrobial phytochemicals produced by plants under attack by bacteria and fungi. Numerous phytoalexins have been identified, including hydrogen peroxide, terpenoids, aromatic acids, oxygenated fatty acids, aliphatic alcohols and polyols. Many of these compounds are not suitable for use in cosmetics. Therefore, the question remains: Can natural systems be used to gently, safely and effectively preserve cosmetics?
Among the decisive criteria for the use of natural preservative concepts in cosmetics are the availability, effectiveness and the toxicological profile of the actives. Another factor to consider is whether the conversion of naturally occurring building blocks offers a possibility to produce either nature-identical compounds or even more effective analogues thereof. Here is where a thorough understanding of natural concepts becomes decisive for the development of active agents that are new and potent, yet mild and sustainable.
The consumer’s perception that natural raw materials are often better tolerated than non-naturally occurring ones in cosmetic applications follows a simple, comprehendible philosophy: the human organism is the product of an evolutionary process lasting millions of years. As is true for all other creatures on earth, human metabolism has adapted to the surrounding chemical environment. As a consequence it is concluded by many consumers that the human body is far more adapted to cope with naturally occurring compounds than with synthetic ones. For example, although both mineral oils and oils of plant origin may be excellent sources of energy if burned, the human body is able to metabolize only oils of plant origin.1,2
This philosophy about the link between human metabolism and the chemical environment is the basis for the trend for natural cosmetics, but for preserving agents it has to be judged very carefully. It would be misleading or even dangerous to assume that antimicrobials from nature are free from any risks to the consumers. Because an intrinsic property of antimicrobial structures is that they can affect living cells, the toxicological evaluation of antimicrobial substances from nature has to be as thorough as for synthetic ones. However, there is little dispute that raw materials from natural origin have significant benefits over synthetic materials with respect to their impact on the environment and the maintenance of resources.
Identifying preserving agents derived from nature is a complex task. Efficacy and safety have to be the first priority because microbiological contamination of cosmetic products can pose substantial threat to the consumer’s health. It is the art of the researcher to identify adequate structures in nature or build them from natural building blocks.
The same may be true for cosmetic applications: although both synthetic and naturally occurring ingredients may have an excellent cosmetic performance, the synthetic ones often exhibiting a better performancethe skin will more easily deal with naturally occurring compounds than with synthetic compounds that cannot be degraded or built into biological structures. Although the biochemical inertness of mineral oils is well known and most of these compounds will not enter the human metabolism, the intake and accumulation of hydrocarbons are regarded as undesirable.1,2 Natural oil components that are identical to the constituents of human cell membranes can be integrated into living cells. Mineral oils, although having a cosmetic function, preferably will be washed away with the next shower.
For the illustration of antimicrobial actives for natural preservation, two examples from organic acids and glyceryl monoesters will show how these concepts apply to cosmetic formulations.
Organic Acids
The widely accepted principle of antimicrobial action of organic acids is illustrated in Figure 2 and explained here. The cell incorporates molecules of the protonated acid through the membrane. Within the cell plasma the acid dissociates and thus changes the pH within the cell.3 The bacterium has to pump out protons permanently and take in sodium ions to maintain the physiological pH of the cell. This energy-consuming process as well as the decreased pH level inside the cell will lead to a decreased rate of reproduction. Because it also lowers the pH outside the cell to a favorable acidic level, the cell makes things worse by favoring the protonated acid side of the acid-base-equilibrium, enabling the membrane to be penetrated by the protonated acid, which is the active species. Finally the process will end with the death of the microorganism.
The nature of the acid is of utmost importance to end up with an efficient active compound. The right compound will be one that ensures biological availability, penetrates the cell membrane and deprotonates within the cell.
If suitable organic acids are available in sufficient amount they will effectively control the growth of microbes in the product. The chosen active compounds should have the following properties:
• Sufficient solubility in the water phase;
• Availability in the protonated form at a given pH in a formulation;
• A structure that permits passage through the membrane; and
• Ready dissociation of the acid within the cell plasma.
For use in self-preserving formulations or in cosmetics making natural claims, there are various compounds, also found in nature, that are in accordance with the requirements of the BDIH, EcoCert and the Organic Soil Association. One commercial product linea of multifunctional additives has, alone or in combination with others, a demonstrated performance against bacteria, yeast and fungi.4 Among the active species within this product line are levulinic acid and anisic acid, which are well-known for their antimicrobial activity and are found in many natural sources.5,6 According to their primary function as very light perfumes they are declared as “fragrance (parfum)” on the ingredients list shown in Formula 1, an exemplary formulation with biological stabilization by organic acids. The function of the glyceryl caprylate, the only other ingredient with an antimicrobial function in Formula 1, will be described next.
Figure 3 shows the results of a challenge test on Formula 1 conducted according to the European Pharmacopoeia (EP). The EP’s criteria regarding the death rate are somewhat stricter than those of the US Pharmacopoeia and the Personal Care Products Council (formerly the Cosmetic, Toiletry, and Fragrance Association).7
Glyceryl Monoesters
A different mode of action is found in another prominent group of multifunctional molecules, the glyceryl monoesters. Some of these multifunctional surfactants have amphiphilic properties and an excellent microbiological performance. The design of the molecules is optimized to bring them into the cell membrane of microbes and destabilize that membrane because of the presence of an incompatible structure such as an incompatible chain length (Figure 4).8,9
The key is to find molecules with suitable structures.10 In addition, the molecules must be soluble and therefore available in the water phase or in the interface between water and oil, and they must be able to penetrate the outer layer of the membrane. Once there, the molecules will deteriorate the stability of the membrane and finally disrupt it completely.11 To enhance the activity against fungi, a faster-acting agent like certain organic acids can be added.
The primary function of these glyceryl monoesters is moisturizing and refatting of the skin. To avoid the need for altering an existing basic formulation, the formulator and the supplier should discuss the additional functions of these raw materials and their possible impact on the formulation.
Formulations for cosmetics making natural claims can be preserved effectively with glyceryl monoesters. As long as they are produced from naturally occurring, sustainable sources, they are in accordance with the requirements of the relevant European certification bodies (BDIH, EcoCert and Organic Soil Association).
These active compounds are not declared as preservatives due to their primary function as moisturizing and refatting agents. With optimum activity is in the pH range of 4.5 to 7, the field of application is very broad. Formula 2 shows a body spray with biological stabilization using glycerol monoesters as the only antimicrobial agent for preservation in the formula. Figure 5 shows the results of a challenge test on this formulation.
Evaluation of Preservatives
For the formulator it is sometimes difficult to determine which preservative system will work best. There are dozens of antimicrobial actives and hundreds of commercial products. There are single compounds and more or less complex blends. The formulator should select from among the ones that are efficient antimicrobials, toxicologically unobjectionable and accepted by consumers.
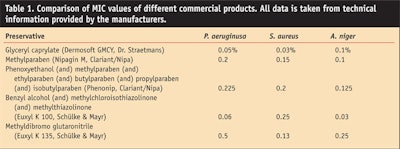
If certain traditional preservatives cannot be used, there are many alternative and natural antimicrobial compounds. A closer look into microbiological data and challenge tests will reveal the usefulness or unreliability of alternative preservatives. The evaluation and comparison of, for example, MIC data can only be a first and rather unreliable step, because this generalized approach takes into account neither the influence of the various raw materials present in a formulation nor chemo-physical hurdles such as solubility and migration of preservatives into the oil phase. The MIC values of some exemplary traditional products are compared to a multifunctional microbial agent in Table 1. Even after studying the MIC or other generalized data, the formulator still must test the preservative system in the targeted cosmetic formulation.
Summary
To meet the growing demand for alternative preservation concepts in cosmetic products making “natural” claims, there is a broad range of active compounds already available to the cosmetic industry. Examples from among organic acids and glyceryl monoesters were described here.
Due to the compounds’ primary properties there is no need to declare them as preservatives, although results of microbiological testing indicate that they provide excellent biological stability. They also allow for a new set of benefits and marketing claims, such as “reduced allergenic load” and “ingredients from sustainable resources.” Indeed, it is difficult to ignore the fact that certain preservatives have suffered from bad press, and more and more cosmetic manufacturers are introducing alternative and natural preservatives in their formulations.12
Natural, sustainable and efficient preservation of cosmetic products can be achieved from the chemical defense mechanisms of nature.
Reproduction of all or part of this article is strictly prohibited.
References
1. Seventeenth Report of the Joint FAO/WHO Expert Committee on Food Additives, World Health Organization Technical Report Series, No 539 (1974)
2. FAO Nutrition Meetings Report Series, No 53 (1974)
3. JJ Kabara, Fatty acids and esters as multifunctional components, In Preservative-Free and Self-Preserving Cosmetics and Drugs, JJ Kabara and DS Orth, eds, New York: Marcel Dekker (1997) p 131
4. JC Meng et al, New antimicrobial mono- and sesquiterpenes from Soroseris hookeriana subsp Erysimoides, Planta Med 66 541–544 (2000)
5. AC Dweck, Natural preservatives, Cosmet Toil 118(8) 45–50 (2003)
6. AC Dweck, An update on natural preservatives, Personal Care 6(4) 11–15 (2005)
7. DS Orth and DC Steinberg, The safety factor in preservative efficacy testing, Cosmet Toil 118(4) 51–58 (2003)
8. G Bergsson et al, In vitro inactivation of Chlamydia trachomatis by fatty acids and monoglycerides, Antimicrob Agents Chemother 42 2290–2294 (1998)
9. DS Orth and JJ Kabara, Preservative-free and self-preserving cosmetics and drugs, Cosmet Toil 113(4) 51–58 (1998)
10. D Smith and W Petersen, The self-preserving challenge, Cosmet Toil 115(5) 67–74 (2000)
11. O Cozzoli, The role of surfactants in self-preserving cosmetic formulas, In Preservative-free and Self-preserving Cosmetics and Drugs, JJ Kabara and DS Orth, eds, New York: Marcel Dekker (1997) pp 110–111
12. J Woodruff, Getting the balance right, SPC (9) 51–54 (2006)