The most important and well-known theory about skin aging is the free radical theory proposed by Harman.1 Reactive oxygen species such as superoxide radicals and hydrogen peroxide and hydroxyl radicals cause oxidative damage to cellular macromolecules, e.g., proteins, carbohydrates, lipids, etc.2 It has been reported that the appearance of the skin is directly related to its antioxidant concentration—i.e., volunteers with higher concentrations of antioxidants in their skin appeared younger, with regard to furrows and wrinkles.3 Therefore, cosmetic scientists are challenged to develop skin care products that deliver antioxidants into the skin.
Coenzyme Q10 and tocopheryl acetate are well-known antioxidants often used in cosmetic products as anti-aging agents.4, 5 Tocopheryl acetate is an ester that can be converted by enzymes in the skin to α-tocopherol, an active antioxidant.6 Moreover, coenzyme Q10 is reported to protect α-tocopherol from photooxidation via a recycling mechanism.7 Incorporation of these two actives into one product can thus provide synergistic anti-aging activity.8
The objective of the present study was therefore to compare the ability of nanoemulsions and emulsions to deliver coenzyme Q10 and tocopheryl acetate into the skin. Further, since additives such as moisturizers—the mainstay for dry, flaky skin treatments—are necessary in anti-aging products, some test formulations also included rice bran oil, cetyl alcohol, glyceryl stearate and a plant extract blend to provide such a benefit. However, because the plant extract blend was fairly new and composed of several chemicals extracted from Asian plants, its effects on the physicochemical and skin penetration properties of formulations were also evaluated.
Nanoemulsions and Emulsions
As noted, in the present study, the delivery of coenzyme Q10 and tocopheryl acetate into newborn pig skin from nanoemulsions was compared with emulsions. While emulsions are one of the most common skin care product forms used, it is questionable whether they deliver high enough concentrations of actives deeper into the skin—especially to the viable epidermis, which is mainly responsible for barrier function and mechanical resistance.9 Generally, the droplet size of emulsions is about 500 nm or greater; however, nanoemulsions are in the range of 20–200 nm, and such technologies, as well as microemulsions and nanostructured lipid carriers (NLC), reportedly are able to overcome the stratum corneum barrier10–15 to carry active compounds through it.
Nanoemulsions are emulsions or dispersions of oil and water stabilized by emulsifying agents. They possess kinetic stability since their small droplet size can reduce the rate of creaming. This effect is demonstrated by Stokes’ law, which shows how the creaming rate decreases as the size of the internal phase decreases.
Stokes’ law : v = d2(ρ - ρ0)g Eq. 1
18η
where v is the velocity of creaming; d is the diameter of the internal droplets; ρ and ρ0 are the densities of the internal phase and dispersion medium, respectively; g is the gravity constant; and η is the viscosity of the dispersion medium.
The formation of nanoemulsions, also referred to as mini-emulsions, ultrafine emulsions and submicron emulsions, generally requires high energy emulsification methods from mechanical devices such as high shear stirrers, high pressure homogenizers or ultrasound generators. An alternative method is to use the chemical energy stored in the nanoemulsion components in a method referred to as low energy emulsification or the phase inversion temperature (PIT) method.16 Today, nanoemulsions are interesting vehicles used to deliver cosmetic substances as well as drugs, such as steroidal and nonsteroidal anti-inflammatory drugs, ceramides and paclitaxel through the skin.17–19
Materials and Methods
Coenzyme Q10 (ubiquinone)a and tocopheryl acetate (vitamin E acetate)b were purchased. A mixed plant extract prepared from various Asian plants, acting as a moisturizer, as well as a mixed emulsifier system were obtained gratisc. Other chemicals used as components in the formulations were purchased from local distributors in Thailand. All chemicals were cosmetic grade and used without further modification. Purified water was used as a vehicle in the formulations. Chemicals used in assay procedures were reagent grade and commercially available.
Sample Preparations
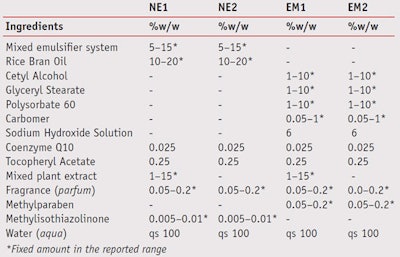
Nanoemulsions and emulsions containing 0.025% w/w coenzyme Q10, 0.25% w/w tocopheryl acetate, with or without mixed plant extract as a moisturizer, were formulated as shown in Table 1. Two nanoemulsions, referred to as NE-1 (with mixed plant extract) and NE-2 (without mixed plant extract), were prepared using low energy emulsification at a constant temperature, modified from that reported by Tadros and co-workers.20 Briefly, the water was added to a solution of 5–15% w/w mixed surfactant system in oil at 50°C under moderate stirring until the dispersion was homogeneous. Once the mixture cooled to room temperature, nanoemulsions were obtained.
To prepare the emulsions, referred to as EM-1 (with mixed plant extract) and EM-2 (without mixed plant extract), the oil and aqueous phases were separately heated to 80°C, after which the water phase was added to oil phase and mixed. In the EM-1 formula, the mixed plant extract was then added at 50°C. Finally, both formulas were continuously mixed for 5 min until the formulations were homogeneous. After the formulations were prepared, the actives were incorporated.
Assaying the Actives
Contents of coenzyme Q10 and tocopheryl acetate in the test formulas were analyzed by high pressure liquid chromatography (HPLC).21 Accurate weights of each sample were mixed with an adjusted volume of tetrahydrofuran using a vortexd for 5 min. Then the mixture was left for 30 min to separate into two layers. A 2.5-mL sample of the upper layer was pipetted, mixed and adjusted to 10 mL with methanol. It was then filtered through a 0.45-µm nylon membrane and the filtrate was finally analyzed by HPLCe. The detector was set at 276 nm and 292 nm for coenzyme Q10 and tocopheryl acetate, respectively, and the analytical columnf used was 5 µm, 4.6 x 150 mm. Methanol: n-hexane (72:28 v/v) was used as the mobile phase at the flow rate of 1 mL/min. This method was validated in that the linearity was found for both actives when the standard concentrations of coenzyme Q10 and tocopheryl acetate ranged from 0.8 to 192 µg/mL and from 19.2 to 192 µg/mL, respectively.
In vitro Skin Permeation and Penetration
Full thickness flank skin weighing 1.4 kg to 1.8 kg was obtained from pigs that died naturally after birthg. The full thickness flank skin was prepared according to a previous report.22 Briefly, the epidermal hair was removed without damaging the skin and excised with a scalpel. The subcutaneous fat and underlying tissue were carefully removed from the dermal surface by surgical scissors. The isolated skin was then mounted on a modified Franz diffusion cell with the stratum corneum facing upward.
A 1.5-g sample of each test formula was then applied in the donor compartment so that the skin was in contact with 1.77 cm2 of the effective area. The receptor compartment was filled with 12 mL of isotonic phosphate buffer (IPB) having a pH of 7.4 and stirred with a magnetic stirrer at 200 rpm. The diffusion cells were connected with a circulating water bath and the temperature was controlled at 37°C. At fixed time intervals of 1, 2, 4, 8 and 12 hr, the amounts of coenzyme Q10 and tocopheryl acetate were determined from two sources—in the receptor fluid and in the skin. For the first portion, the amounts of actives were evaluated by HPLC as previously described. For the second portion, the amounts of actives were elucidated according to the following assay method.
Determination of Actives in Pig Skin
The isolated skin from the permeation and penetration studies was cleaned by wiping it with cotton soaked with IPB to remove excess preparation before determining the amounts of coenzyme Q10 and tocopheryl acetate at the fixed intervals. The cleaned skin was then cut into small pieces and further minced with IPB using a blender. The calibration curve of coenzyme Q10 and tocopheryl acetate in skin was constructed according to a previous report.23
To validate the amounts of coenzyme Q10 and tocopheryl acetate extracted from the skin, the control samples were prepared by spiking the homogenous blank skin with a known concentration of the active compounds. The skin was homogenized with twice-fold amounts, by volume, of ice-cold IPB by a homogenizerh at 24,000 rpm for 5 min. The homogenate was screened through a gauze filter. Known concentrations of coenzyme Q10 and tocopheryl acetate, ranging from 0.8 µg/mL to 200 µg/mL, were added in the obtained filtrate and applied to a centrifugej with the relative centrifugal force of 12,000 × g for 20 min.
The actives were extracted from the aqueous solution using n-hexane. The upper layer was pipetted and n-hexane was removed using an automatic speed vac concentratork. The obtained precipitate was dissolved in 0.5 mL of the mobile phase and the amounts of the actives were determined by HPLC. The extraction recovery was determined by computing the ratios of the amounts of the actives extracted from spike skin and the active solutions at the identical concentrations. The validation data indicated that linearity was observed when the standard concentrations of both actives were in the range of 0.8 µg/mL to 200 µg/mL.
Stability Tests for NE1 and EM1
Stability tests were conducted under freeze-thaw conditions for six cycles. In each cycle, each sample was kept at -5°C for 24 hr and at 45°C for 24 hr. In addition, the samples were evaluated for one-month stability under three conditions—i.e., in clear, glass containers at 50°C; in clear, glass containers under a fade lamp; and in dark containers at 25°C. Changes in physical appearance and the active contents were determined. The physical characteristics of formulations such as color and odor were sensory observed. The pH values were determined in triplicate by a pH meterm. Particle size was measured in triplicate using a photon correlation spectroscopy (PCS) techniquen. The contents of the actives were again assayed by HPLC.
Results and Discussion
Figures 1 and 2 demonstrate the HPLC chromatograms in a) a standard solution, b) an extract of a formulation, c) a skin extract after application for 12 hr, and d) the receptor fluid of coenzyme Q10 and tocopheryl acetate, respectively. The peak of coenzyme Q10 was depicted at a retention time about 9.7 min, and that of tocopheryl acetate at about 4.2 min, as demonstrated in Figures 1a and 2a, respectively. These two peaks were observed in the extract of the formulation and of the applied skin as seen in Figures 1b, 2b, 1c and 2c, respectively. No peaks of actives were found in the receptor fluid, illustrated in Figures 1d and 2d. The results indicated that coenzyme Q10 and tocopheryl acetate could accumulate in the skin but could not reach the receptor fluid, representative of blood circulation.
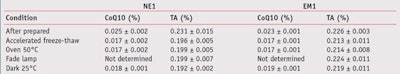
The contents of coenzyme Q10 and tocopheryl acetate in all formulations were acceptable since the percentage of labeled amount for coenzyme Q10 was 92% to 101% and that of tocopheryl acetate was 90% to 106%. Table 2 presented that the amounts of delivered coenzyme Q10 into the skin ranged as NE1 > NE2 > EM2 ≈ EM1, while those of tocopheryl acetate ranged as NE1 > NE2 > EM1 > EM2. On average, nanoemulsions could enhance skin penetration of coenzyme Q10 by approximately 1.2–1.5 fold, and tocopheryl acetate by about 2.1–2.6 fold higher than emulsions. This enhancement effect could be explained by the nanosized droplets dispersed in the continuous phase of the nanoemulsions, which can move easily through the stratum corneum and carry the actives through the skin barrier.
It is interesting to note that the mixed plant extract did not significantly affect the penetration of the actives (p > 0.05); however, the means of penetrated actives from the formulations with mixed plant extract (NE1, EM1) seemed to be higher than those from the formulations without mixed plant extract (NE2, EM2) due to moisturizing or hydration effect.
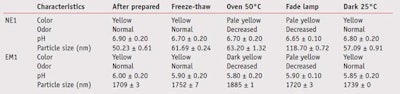
The results of physical stability study of NE1 and EM1 performed under different conditions are presented in Table 3. While the structures of the studied nanoemulsions and emulsions were identical—i.e., oil droplets dispersed in aqueous medium, the difference was their size. As Table 3 shows, the particle size of the nanoemulsions was in the range of 50–119 nm and that of the emulsions was in the range of 1709–1885 nm.
Characteristics of NE1 and EM1 were not changed when stored in dark containers at 25°C. High temperature and fade lamp affected the characteristics of both samples. Fade light was found to be a major parameter causing a significant increase in the particle size of the nanoemulsions. In addition, fade light caused a significant increase in the particle size of blank nanoemulsions without any actives (data not shown). It could be explained that fade light catalyzed the coalescence of the internal droplets of nanoemulsions. This phenomenon obviously was not found in emulsions, although the reason was not clear. However, it could be because emulsions had their own low free surface energies.
The amounts of actives in all formulations tended to decrease after being stored in the stability-tested conditions, as shown in Table 4. It was found that the amount of coenzyme Q10 in both formulation types significantly decreased in all conditions of stability testing (p < 0.05). On the other hand, the amount of tocopheryl acetate in nanoemulsions only significantly decreased with stability testing. Tocopheryl acetate formulated in emulsions was more stable than in nanoemulsions. It was hypothesized that the preparation process could affect the stability of tocopheryl acetate.
Conclusions
With respect to in vitro skin penetration studies, coenzyme Q10 and tocopheryl acetate loaded in nanoemulsions could penetrate and accumulate in the skin in higher concentrations than when loaded in emulsions. Further, all studied formulations did not pose systemic effects since no actives were found in the receptor fluid, which represents blood circulation. However, this study was conducted with intact newborn pig skin, so the results revealed the test formulations were safe when applied to normal skin. The stability data suggested that the products should be stored in dark conditions at 25°C, although enhancement of the chemical stability of the nanoemulsions was required.
The described work suggests nanoemulsions may be a preferred formulation type to deliver skin care actives, e.g., antioxidants and skin-whitening agents, when the target site is the viable epidermis. Nanotechnology formulations including microemulsions, NLCs and nanoemulsions provide an interesting option for skin penetration enhancement. However, it should be noted that their toxicity must be considered, and it is the responsibility of cosmetic scientists to thoroughly test formulations before their use to ensure that the actives cannot penetrate into the bloodstream. Further, the somewhat complicated preparation process could impact the stability of the actives or increase the cost.
Acknowledgements: The authors wish to acknowledge that this research received financial support from International Laboratories Corp., Ltd., of Bangkok, Thailand, to fulfill the study.
References
Send e-mail to [email protected].
1. D Harman, Aging: A theory based on free radical and radiation chemistry, J Gerontol 11, 298–300 (1956)
2. B Chakravarti and DN Chakravarti, Oxidative modification of proteins: Age-related changes, Gerontol 53 3, 128–139 (2006).
3. M Darvin and J Lademann, Antioxidants in the skin: Dermatological and cosmeceutical aspects, in Dermatologic, Cosmeceutic, and Cosmetic development, KA Walters and MS Roberts, eds, Informa Healthcare, New York (2007) pp 373–384
4. J Hatanaka, Y Kimura, A Lai-Fu, S Onoue and S Yamada, Physicochemical and pharmacokinetic characterization of water-soluble coenzyme Q(10) formulations, Int J Pharm 363 1–2 112–117 (2008)
5. Y Shindo, E Witt, D Han, W Epstein and L Packer, Enzymic and non-enzymic antioxidants in epidermis and dermis of human skin, J Invest Dermatol 102 1 122–124 (1994)
6. JJ Thiele, S Ekanayake-Madiyanselage and SN Hsieh, Cosmeceutical vitamins: Vitamin E, in Cosmeceuticals, ZD Draelos, ed, Elsevier Saunders, Philadelphia (2005) pp 43–56
7. L Ernster and G Dallner, Biochemical, physiological and medical aspects of ubiquinone function, Biochim Biophys Acta 127 1 195–204 (1995)
8. L Baumann, How to prevent photoaging, J Invest Dermatol 125 4 xii–xiii (2005)
9. K Higaki, C Amnuaikit and T Kimura, Strategies for overcoming the stratum corneum chemical and physical approaches, Am J Drug Deliv 1 3 187–214 (2003)
10. O Sonneville-Aubrun, JT Simonnet and F L’Alloret, Nanoemulsions: A new vehicle for skin care products, Adv Colloid Interface Sci 108–109, 145–149 (2004)
11. P Boonme, Applications of microemulsions in cosmetics, J Cosmetic Dermatol 6 4 223–228 (2007)
12. V Teeranachaideekul, P Boonme, EB Souto, RH Müller and VB Junyaprasert, Influence of oil content on physicochemical properties and skin distribution of Nile red-loaded NLC, J Control Release 128 134–141 (2008)
13. VB Junyaprasert, V Teeranachaideekul, EB Souto, P Boonme and RH Müller, Q10-loaded NLC versus nanoemulsions: Stability, rheology and in vitro skin penetration, Int J Pharm 377, 207–214 (2009)
14. P Boonme, Uses of microemulsions as novel vehicles in skin care products, HPC Today 3 2 18–20 (2009)
15. P Boonme, VB Junyaprasert, N Suksawad and S Songkro, Microemulsions and nanoemulsions: Novel vehicles for whitening cosmeceuticals, J Biomed Nanotech 5 4 373–383 (2009)
16. EB Souto, V Teeranachaideekul, P Boonme, RH Müller and VB Junyaprasert, Lipid-based nanocarriers for cutaneous administration of pharmaceutics, in Encyclopedia of Nanoscience and Nanotechnology, HS Nalwa, ed, Los Angeles: American Scientific Publishers, in press (2010)
17. DI Friedman, JS Schwarz and M Weisspapi, Submicron emulsion vehicle for enhanced transdermal delivery of steroidal and nonsteroidal anti-inflammatory drugs, J Pharm Sci 84, 324–329 (1995)
18. E Yilmaz and HH Borchert, Design of a phytosphingosine-containing positively-charged nanoemulsions as a colloidal carrier system for dermal application of ceramides, Eur J Pharm Biopharm 60 91–98 (2005)
19. S Khandavilli and R Panchagnula, Nanoemulsions as versatile formulations for paclitaxel delivery: Peroral and dermal delivery studies in rats, J Invest Dermatol 127 154–162 (2007)
20. TF Tadros, P Izquierdo, J Esquena and C Solans, Formation and stability of nano-emulsions, Adv Colloid Interface Sci 108–109, 303–318 (2004)
21. J Karpi´nska, B Mikolu´c, R Motkowski and J Piotrowska-jastrz˛ebska, HPLC method for simultaneous determination of retinol, α-tocopherol and coenzyme Q10 in human plasma, J Pharm Biomed Anal 42 2 232–236 (2006)
22. S Songkro, Y Purwo, G Becket and T Rades, Investigation of newborn pig skin as an in vitro animal model for transdermal drug delivery, STP Pharma Sci 13 2 133–139 (2003)
23. K Higaki, M Asai, T Suyama, K Nakayama, K Ogawara and T Kimura, Estimation of intradermal disposition kinetics of drugs: II factors determining penetration of drugs from viable skin to muscle layer, Int J Pharm 239 129–141 (2002)