Atopic dermatitis (AD) is a chronic inflammatory skin disease that most commonly occurs during early infancy and childhood and is characterized by a chronically relapsing course where symptoms of AD are ameliorated and aggravated repeatedly. Although the pathogenesis of AD is not fully understood, it has been reported that it is associated with multiple immunologic abnormalities.1,2 The most prominent findings of immune dysfunction are the high level of immunoglobulin E (IgE) in peripheral blood and increased IgE production by B cells. In AD, T helper 2 (Th2) cells are mainly activated and then secrete interleukin-4 (IL-4), interleukin-5 (IL-5) and interleukin-10 (IL-10).1, 3, 4
Symptoms of AD induced by signal transduction pathways of IgE, IL-4, IL-5 or IL-10 are associated with inflamed skin damage as well as skin dryness. Accordingly, the generally accepted prescription includes moisturizers and topical steroids that maintain a level of moisture in the skin and suppress inflammatory reaction, respectively. However, treating AD over time with topical steroid hormones has been known to induce adverse side effects.5 In addition, nonsteroid therapeutic treatments including cyclosporin A and tacrolimus have been reported to induce cutaneous T cell lymphoma, fever, extreme rises in serum alkaline phosphatase in children, enhanced irritation and relapsing Kaposi’s varicelliform eruption.6–10 Therefore, much recent work has focused on the development of therapeutic agents that maximize anti-inflammatory effects while minimizing side effects.
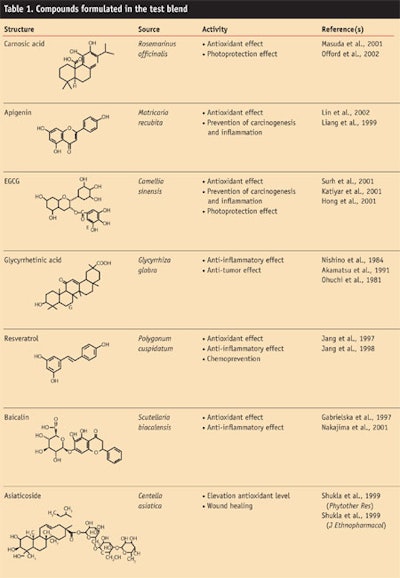
As a first step, the authors selected chemicals with known anti-inflammatory and antioxidant mechanisms to prepare a variety of formulations. Subsequently, candidate formulations exhibiting high anti-inflammatory effects using a nuclear factor kappa-B (NF-κB) luciferase reporter were screened. The results of the screening led to the development of a blend consisting of seven medicinal plant componentsa. These components included: carnosic acid from Rosmarinus officinalis; apigenin from Matricaria recubita; epigallocatechin-3-gallate from Camellia sinensis; glycyrrhetinic acid from Glycyrrhiza glabra; resveratrol from Polygonum cuspidatum; baicalin from Scutellaria baicalensis; and asiaticoside from Centella asiatica (see Formula 1 and Table 1). In the present study, the authors investigated in vitro the anti-inflammatory effects of this preparation for its potential to treat AD.
Materials and Methods
To set up the test for anti-inflammatory effects of the plant blend, researchers first obtained the following materials: lipopolysaccharideb (LPS), phytohematoaglutininc, IL-2 luciferase reporter DNA,11 IL-8 ELISA kitd, TNF-α ELISA kite and NF-κB DNAf. Three formulations containing the preparation were then prepared using dimethyl sulfoxide as the solvent;12 these included a face/body wash containing 1% test blend(Formula 2), a liposome lotion containing 1.5% test blend (Formula 3), and a liposome serum containing 3% test blend (Formula 4). The liposome lotion and serum were manufactured in a liposome base using the one-step homogenization technique as described by Brandl et al.13 The face/body wash was a general liquid soap with a low detergent level.
Cell lines and cell culture: THP-1 human monocyte cells and Jurkat human leukemia cells were cultured in a bicarbonate buffering system with variations in the amounts of amino acids and vitaminsg including 10% fetal bovine serum and 1x antibiotic solution. HaCaT cells were cultured in Dulbecco’s modified eagle’s medium (DMEM) including 10% fetal bovine serum and 1x antibiotic solution. Cells were incubated at 37°C in a 5% CO2 incubator.14, 15
Transient cell transfection and luciferase reporter gene assay: THP-1 cells were transiently transfected with 2 mg of the firefly luciferase reporter gene under the control of NF-κB responsible element and 0.2 mg of renilla luciferase expression vector driven by thymidine kinase promoterh by superfect reagent. The transfected cells were transferred to 6 well plates and incubated for 24 hr at a density of 8 X 105 cells per mL.
After 24 hr, the cells were further cultured in the presence or absence of LPS and the test blend for 5 hr. Luciferase activity was determined using the high-throughput analysis of mammalian cells containing genes for firefly and renilla luciferasesj and a luminometerk, and were expressed as a ratio of NF-κ B-dependent firefly luciferase activity divided by control thymidine kinase renilla luciferase activity (relative luciferase unit). Results were confirmed by three independent transfections. To assay for IL-2 promoter activity, Jurkat T cells were transfected with 2 µg of reporter plasmids using a transfection reagentm. After 24 hr, cells were activated by treatment with PHA (10 μg/mL) and harvested. Luciferase activity was determined in triplicate for each experiment with a lumino-meterj.16, 17
Measurement of cytokine production: Cell supernatants were analyzed for IL-8 and TNF-α by using commercially available ELISA kits with sensitivities of 3 and 1 pg/mL, respectively.
Cytotoxicity assay: HaCaT cells were cultured in DMEM including 10% fetal bovine serum and 1x antibiotic solution. Cells were incubated at 37°C in a 5% CO2 incubator. Cells were seeded on 24 well plates and drug treatment began 24 hr after seeding. General viability of cultured cells was determined by reduction of 3-(4,5-dimety-2-thiazoyl)-2,5-dipheny-2H-ltetrazolium bromide (MTT) to formazan. After incubation of HaCaT cells treated with various concentrations of the test blend for 24 hr at 37°C in 5% CO2 atmosphere, an MTT assay was performed. MTT (1 mg/mL in PBS) was added to each well, 1/10 volume of media. Cells were incubated at 37°C for 3 hr and harvested by centrifugation, after which dimethyl sulfoxide was added to dissolve the formazan crystals. The absorbance was then measured at 570 nm with a spectrophotometern.
Statistical analysis: Non-parametric, one-way analysis of variance (Kruskal Wallis test) and the Mann-Whitney test were used for statistical analysis. Differences between groups were considered significant at p < 0.05 (*).
AD-mitigating Effects
Thirty children with mild AD, including 11 boys and 19 girls, ages 6–15, participated in an in vivo study. Written consent was obtained in each case and three variations of the test blend preparations were administered using the following instructions:
1. Face and body wash was to be used when taking a bath with lukewarm water for approximately 10 min each evening. Liposome lotion was then applied to the entire body immediately after removing excess water from bathing.
2. Liposome serum was additionally applied to severely dry skin or severely affected AD lesions twice daily—once in the morning and once in the afternoon—irrespective of the bathing. Topical or systemic uses of corticosteroids were strictly prohibited during the study period except oral antihistamines offered to control severe pruritus.
Clinical evaluation was performed objectively on the symptoms and degree of AD using the Eczema Area Severity Index (EASI) as presented by
J. M. Hanifin et al.18 Pruritus was evaluated using a 10-point visual analog scale. Transepidermal water loss (TEWL) was measured before treatment, at two weeks of treatment, and at four weeks after treatment on the abdomen and right antecubital fossa. In addition, a patch test was performed on the back or forearm in 10 patients to check for any irritative or allergic effects.
For statistical analysis, Kruskal-Wallis one-way ANOVA was used to compare the changes of EASI, TEWL and pruritus according to the treatment period. A p value of less than 0.05 was considered to be statistically significant. In addition, a dimethyl sulfoxide-only group was used as a placebo (negative control).
LPS-induced NF-κB activation: NF-κB is a protein transcription factor that was first identified by Sen and Baltimore;19 it enhances the transcription of a variety of genes including cytokines and growth factors, adhesion molecules, immunoreceptors and acute-phase proteins. NF-κB reportedly has been involved in maximal transcription of many cytokines, including TNF-α, IL-1, IL-6 and IL-8, which are considered to be important in the generation of acute inflammatory responses.
As a preliminary step to determine whether the test blend affects cytokine production, a NF-κB luciferase assay was performed. As shown in Figure 1, LPS increased NF-κB reporter activity fivefold, whereas the test blend inhibited LPS-induced NF-κB reporter activity in a dose-dependent manner. In the experiment, THP-1 cells were transfected with NF-κB-luciferase using superfect. After incubation for 24 hr, cells were stimulated for 14 hr with LPS, harvested and lysed. Supernatants were assayed for luciferase activity.
Luciferase activity was determined three times in duplicate for each experiment. All values were significant(*p < 0.01) when compared with values for LPS alone. LPS-induced NF-κB reporter activity was diminished by one half when the test blend was treated at 1% concentration, indicating that IC50 of the blend is 1%. This result suggests the possibility that the test blend may be involved in blocking the production of proinflammatory cytokines.
LPS-induced production of IL-8 and TNF-α: The effect of the test blend on the production of IL-8 and TNF-α was also examined. Cells (106) were incubated with nothing, with LPS (100 ng/mL), or with LPS plus the test blend for 24 hr. Afterwards, supernatants were assessed for IL-8 by ELISA (Figure 2) or for TNF-α (Figure 3). Data is presented as the mean ± standard deviation of four separate experiments. All values were significant (*p < 0.01) compared with values for LPS alone. The test blend significantly reduced the production of IL-8 and TNF-α dose-dependently, confirming that the blend inhibits production of IL-8 and also suggesting the anti-inflammatory function of the test blend through inactivation of NF-κB promoter. These findings were similar to those found in Figure 1.
IL-2 production in Jurkat T cells: To explore the involvement of the test blend in T cell activation and proliferation, IL-2 luciferase reporter system was used. As shown in Figure 4, Jurkat T Cells were transfected with IL-2-luciferase using superfect. After incubation for 24 hr, cells were stimulated for 14 hr with PHA, harvested and lysed. Supernatants were assayed for luciferase activity. Luciferase activity was determined three times in duplicate for each experiment. All values were significant (p < 0.01), compared with values of PHA alone. PHA distinctly activated IL-2 reporter activity by tenfold. However, this increased reporter activity was inhibited by the test blend, indicating it has the potential to inhibit T cell activation and proliferation in Jurkat T cells.
Cytotoxic property in HaCaT cells: Next, the cytotoxic effects of the test blend were examined on HaCaT cells, human keratinocyte cell lines (see Figure 5). In this experiment, HaCaT cells were cultured for 24 hr in a medium with or without the test blend. The cellular cyto-
toxicity was determined according to a rapid colorimetric MTT assay and expressed as the mean ± S.D. While the test blend at concentrations lower than 5% showed almost 100% cell viability, the tested blend at 10% showed a more than 80% cell viability of HaCaT cells. Thus, in HaCaT cells, the test blend did not show a significant cytotoxic effect (data not shown). This suggests the blend has low cytotoxic properties.
Clinical Study
In a clinical study, a statistically significant reduction of EASI score was observed at 2 weeks (1.63 ± 00.60) and at 4 weeks (0.61 ± 0.48) after treatment with the test blend, compared with the EASI score prior to treatment (2.60 ± 0.57)
(p = 0.001).
Pruritus also decreased significantly at 2 weeks (4.47 ± 0.53) and at 4 weeks (3.20 ± 0.49) after treatment (p = 0.022), compared with scores before treatment (5.13 ± 0.46). The effect of the test blend on TEWL also was studied on the antecubital fossa and on the abdomen. TEWL decreased after treatment, both on the antecubital fossa and abdomen, although only on the abdomen showed a statistically significant decrease (p = 0.01) 2 weeks (17.31 ±
1.85 g/m2/h) and 4 weeks (13.46 ±
2.42 g/m2/h) after treatment, when compared with scores prior to treatment (22.81 ± 2.18 g/m2/h).
In regard to the patch testing, consistent with in vitro cytotoxic assays, no erythema, burning or pruritus reactions were observed in any patients based on the 2- and 4-day readings. Only one patient complained of pruritus immediately after application of liposome lotion and this patient dropped out of the study. The face/body wash containing the test blend and emollients was thus considered to be effective and safe for use in AD patients.
Discussion and Conclusions
As previously mentioned, despite the effectiveness of steroid hormones on AD, they have been known to induce side effects such as skin atrophy, dilation of blood vessels, depigmentation and so on.5 Therefore, this study aimed to evaluate whether a blend of medicinal plant extracts could be used as an alternative or adjuvant therapeutic agent to treat AD. To this end, in vitro and in vivo experiments were performed.
In this report, the characterization of a test blend to act as an anti-inflammatory and AD-mitigating agent has been described and several benefits demonstrate its possibility as a therapeutic agent to treat AD. The blend inhibited LPS-induced NF-κB activation and reduced the LPS-induced production of IL-8 and TNF-α. In addition, it inhibited IL-2 production in Jurkat T cells, demonstrating anti-inflammatory effects and the involvement in blocking T cell-mediated immune responses.
In in vivo studies, after treatment with the test blend, a statistically significant reduction of EASI score was observed, as well as a decrease of pruritus and TEWL both on the antecubital fossa and abdomen. In addition, patch testing showed no reactions in any subjects, suggesting the test blend can safely be applied to human skin. Based on these results, the authors conclude that this medicinal plant blend can be safely applied to patients with AD. However, an understanding of the precise molecular mechanisms by which the blend improves AD remains incomplete. While cyclosporin A and tacrolimus, which inhibit IL-2 production in Jurkat T cells, are known to block PLC gamma-mediated Ca2+ signaling,20–23 rosmarinic acid inhibits TCR-proximal p56lck-mediated signaling.24–26 In light of the IL-2 luciferase assay shown in Figure 4, the test blend may inhibit the Ca2+ signaling mediated by calcineurin or the p56lck-mediated signaling that is known to play an important role in
T cell activation and proliferation.27–31
Various agents that induce inflammation are known to activate
NF-B.32,33 In addition, several genes that are involved in inflammation are regulated by NF-κB.34–36 Therefore, it was hypothesized that NF-κB promoter can be employed as a marker to screen anti-inflammatory candidates. The finding that the test blend inhibits LPS-induced NF-κB activation implicates its use in anti-inflammatory signaling and also suggests that its action mechanism may be mediated through suppression of the recruitment of signaling molecules involved in NF-κB activation.
Until now, a limited number of proteins involved in the NF-κB activation pathway have been identified, including TNFRSF1A-associated via death domain, NF-kB-inducing kinase (NIK), mammalian mitogen-activated protein kinase and Iκ B kinase.37–40 The interaction between these molecules has previously been elucidated as critical in NF-κB activation signaling.41, 42
Therefore, it is likely that the test blend may block cross-talk among these signaling molecules.
As previously mentioned, the test preparation consists of seven compounds and their individual and/or combined effects are currently unknown. Studies to elucidate possible reciprocal interactions between them are in progress. Overall, however, the results procured thus far indicate this test blend can be used as an effective topical treatment for AD.
Reproduction of all or part of this article strictly is prohibited.
To get a copy of this article or others from a searchable database, visit the C&T magazine articles archives at: www.CosmeticsandToiletries.com/magazine/pastissues.
References
1. KD Cooper, Atopic dermatitis: Recent trends in pathogenesis and therapy, J Invest Dermatol 102 128–137 (1968)
2. JD Bos et al, Immune dysregulation in atopic eczema, Archives of Dermatol 128 1509–1512 (1992)
3. TR Mosmann et al, Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins, J Immunol 136 2348–2357 (1986)
4. L SH et al, Studies on serum IL-4 as a marker of symptom in patients with atopic dermatitis, Korean J Dermatol 36 95–102 (1998)
5. P Venge, Eosinophil and neutrophil granulocytes, Allergy 36 95–102 (1993)
6. B Kirby, Cutaneous T-cell lymphoma developing in a patient on cyclosporin therapy, J Am Acad Dermatol 47(2) S165–S167 (2002)
7. MD Thomas et al, Fever associated with cyclosporin for atopic dermatitis, Br Med J 317 1291 (1998)
8. T Van Meurs, Extreme rises in serum alkaline phosphatase in children with atopic dermatitis after intervention treatment with cyclosporin A, Pediatric Dermatol 15(6) 483 (1998)
9. M Ambo, Relapsing Kaposi’s varicelliform eruption and herpes simplex following facial tacrolimus treatment for atopic dermatitis, Acta Dermato-Venereologica 82(3), 224–225 (2002)
10. M Fuchs et al, Tacrolimus enhances irritation in a 5-day human irritancy in vivo model, Contact Dermatitis 46(5) 290–294 (2002)
11. BC Young, KK Chan and Y Yungdae, An adapter protein interacting with the SH2 domain of p56lck, is required for T cell activation, J Immunology 163, 5242–5249 (1999)
12. L Jongsung et al, Evaluation of the anti-inflammatory and immunomodulatory effects of BSASM using in vitro experiments, Korean J Pharmacognosy 34(3) 228–232 (2003)
13. M Brandl et al, Three-dimensional liposome networks: Freeze fracture electron microscopical evaluation of their structure and in vitro analysis of release of hydrophilic markers, Advanced Drug Delivery Reviews 24 161–164 (1997)
14. H Nishino et al, Antitumor-promoting activity of glycyrrhetic acid in mouse skin tumor formation induced by 7,12-dimethylbenz[a]anthracene plus teleocidin, Carcinogenesis 5 1529–1530 (1984)
15. N Za et al, Jurkat cell proliferative activity is increased by luteinizing hormone-releasing hormone, J Endocrinology 153 241–249 (1997)
16. TM Williams et al, Advantages of firefly luciferase as a reporter gene: Application to the interleukin-2 gene promoter, Analytical Biochem 176 28–32 (1989)
17. PR Wenner et al, Detection and quantification of cyclosporine in body fluids using an interleukin-2 reporter-gene assay, J Immunological Methods 201 125–135 (1997)
18. JM Hanifin, M Thurston, M Omoto, R Cherill, SJ Tofte and M Graeber, The eczema area and severity index (EASI): Assessment of reliability in atopic dermatitis. EASI Evaluator Group, Experimental Dermatol 10 11–18 (2001)
19. R Sen and D Baltimore, Multiple nuclear factors interact with the immunoglobulin enhancer sequences, Cell 46 705–716 (1986)
20. GA Dos and EM Shevach, Effect of cyclo-sporin A on T cell function in vitro: The mechanism of suppression of T cell proliferation depends on the nature of the T cell stimulus as well as the differentiation state of the responding T cell, J Immunology 29 2360–2367 (1982)
21. M Eichhorn et al, IL-2 can enhance the cyclosporin A-mediated inhibition of Theileria parva-infected T cell proliferation, J Immunology 144 691–698 (1990)
22. MJ Tocci et al, The immunosuppressant FK506 selectively inhibits expression of early T cell activation genes, J Immunology 143 718–726 (1989)
23. SS Banerji et al, The immunosuppressant FK-506 specifically inhibits mitogen-induced activation of the interleukin-2 promoter and the isolated enhancer elements NFIL-2A and NF-AT1, Mol Cellular Biol 11 4074–4087 (1991)
24. J Won et al, Rosmarinic acid inhibits TCR-induced T cell activation and proliferation in an Lck-dependent manner, European, J Immunology 33 870–879 (2003)
25. MA Kang, Rosmarinic acid inhibits Ca2+-dependent pathways of T-cell antigen receptor-mediated signaling by inhibiting the PLC-gamma 1 and Itk activity, Blood 101 3534–3542 (2003)
26. SY Yun et al, Synergistic immunosuppressive effects of rosmarinic acid and rapamycin in vitro and in vivo, Transplantation 75 1758–1760 (2003)
27. SJ Anderson and RM Perlmutter, A signaling pathway governing early thymocyte maturation, Immunology Today 16 99–105 (1994)
28. TJ Molina et al, Profound block in thymocyte development in mice lacking p56lck, Nature 357 161–164 (1992)
29. M Hatakeyama et al and Taniguchi. Interaction of the IL-2 receptor with the src-family kinase p56lck: Identification of novel intermolecular association, Science 252 1523–1528 (1991)
30. Y Minami et al, Association of p56lck with IL-2 receptor beta chain is critical for the IL-2-induced activation of p56lck, The EMBO Journal 12 759–768 (1993)
31. JD Marth et al, Neoplastic transformation induced by an activated lymphocyte-specific protein tyrosine kinase (pp56lck), Mol Cellular Biol 8 540-550 (1988)
32. C Giuliani et al, NF-kappaB transcription factor: Role in the pathogenesis of inflammatory, autoimmune, and neoplastic diseases and therapy implications, Clinical Therapeutics 152 249-253 (2001)
33. R Donadelli et al, Protein traffic activates NF-kappaB gene signaling and promotes MCP-1-dependent interstitial inflammation, Am J Kidney Disease 36 1226-1241 (2000)
34. AV Miagkov et al, NF-kappaB activation provides the potential link between inflammation and hyperplasia in the arthritic joint, Proceedings of the National Academy of Sciences of the United States of America 95 13859-13864 (1998)
35. W Zheng-Ming et al, Chemokines are the main proinflammatory mediators in human monocytes activated by Staphylococcus aureus, peptidoglycan, and endotoxin, J Biol Chem 275(27) 20260–20267 (2000)
36. R Benjamin et al, Induction of proinflammatory cytokines by a soluble factor of Propionibacterium acnes: Implication for chronic inflammatory acne, Infection and Immunity 63(8) 3158–3165 (1995)
37. M Naofumi et al, Molecular mechanism of interleukin-8 gene expression, J Leukocyte Biol 56 554–558 (1994)
38. M Morgan et al, Nuclear and cytoplasmic shuttling of TRADD induces apoptosis via different mechanisms, J Cell Biol 157 975–984 (2002)
39. X Lin et al, Molecular determinants of NF-kappaB-inducing kinase action, Mol Cellular Biol 18 5899–5907 (1998)
40. AH Bild et al, MEKK1-induced apoptosis requires TRAIL death receptor activation and is inhibited by AKT/PKB through inhibition of MEKK1 cleavage, Oncogene 21 6649–6656 (2002)
41. D Krappmann et al, The I kappa B kinase (IKK) complex is tripartite and contains IKK gamma but not IKAP as a regular component, J Biol Chem 275 29779–29787 (2000)
42. G Takaesuet al, TAB2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway, Mol Cell 5 649–658 (2000)