Knowledge of skin biology and the chemistry of UV filters and formulations has improved dramatically in the last 20 years, enabling cosmetic chemists to formulate unique and effective sunscreen products and delivery systems. Moreover, efficacy testing methods for these products have become almost standardized worldwide, even though the United States and the European Union may differ in the protocols they use for these methods.
Thus in normal skin, sunscreen formulations can be applied topically to reduce the negative effects of sun exposure. However, for various reasons, they are inadequate or inappropriate in the case of acne-prone skin.
Many of the numerous conditions that contribute to acne are actually accentuated during the summer months when exposure to sunlight is maximized. Thus, how can sunscreen products be formulated and used in a way that maximizes their acne-control benefits in the setting of acne-prone skin? One answer lies in a uniquely formulated sunscreen product intended to exhibit activity on acne in skin. The benefits are demonstrated in clinical studies reported in this article.
Sun and Acne
Although the main reason for the development of acne is increased sebum production, three other factors must be considered: ductal cornification, bacterial colonization of the pilosebaceous duct and inflammation.1 Thus propionibacteria, which form part of the resident cutaneous microflora, are thought to play an important role in the pathogenesis of inflamed lesions. Sebaceous follicles in which comedogenesis is occurring along with increased amounts of sebum are characterized by marked overgrowth of Propionibacterium acnes and that proliferation can be accompanied by generation of pro-inflammatory molecules and subsequent inflammation.
In addition, lipases and other enzymes from P. acnes hydrolyze sebum triglycerides into free fatty acids that are both comedogenic and pro-inflammatory. Furthermore, rupture of the follicle, with the release of the follicular contents to the surrounding connective tissues, accelerates progression of inflammatory changes.
Kligman, Katz2 and Kanaar3 have shown that certain sebaceous lipids, especially squalene and free fatty acids, are comedogenic. Meanwhile, Morganti, Fabrizi and co-workers4–7 have demonstrated that higher concentrations of linoleate and soluble azelaic acid may improve the treatment of acne.
The sun also has a strong effect on patients with acne vulgaris.8 Infrared and ultraviolet radiations (UVR) increase both comedone formation and skin surface lipids from sebaceous glands and the epidermis.9 In fact, comedone formation that results from irritation caused by specific free fatty acids and is aggravated by UV rays induces hyperkeratosis and at the same time increases these fatty acids. This is probably the consequence of increased lipase activity caused by the spread of microbial flora.10
Generally speaking, well-known anti-acne pharmaceutical products that are often based on benzoyl peroxide or retinoic acid cannot be used on sun-exposed skin because of their sun-sensitization properties. Their use is therefore suspended during the summer and resumed in the fall. During summer it is important to use sunscreen formulations that are also active on the acne pathogenesis; during colder months, it is important to continue with the winter drug therapy regimen.
On one hand, while most commercial sunscreens do not cause acne, they are formulated only to protect skin from UV damage. On the other hand, during sunny months patients with acne vulgaris suspend their therapy because of the sun-sensitization properties of all the drugs used for this purpose.
From this conflict arises the need to formulate a sunscreen product specific for acne-prone skin.
Sunscreen Product for Acne-prone Skin
A sunscreen product that also provides anti-acne treatment should be lipid-free, nonallergenic, noncomedogenic, photochemically stable and cosmetically acceptable.11–12 The recommended sunscreen actives are titanium dioxide and ethylhexyl methoxycinnamate; the recommended vehicle is based on phosphatidylcholine and hydrosoluble azelaic acid.5,6,11,12 These last two compounds, known for their proven efficacy on acne-prone skin, are also capable of decreasing sebum production.13 The first two compounds were selected because they are noncomedogenic and commonly used sunscreen agents.
One approach to formulating a sunscreen for acne-prone skin is to use a topical sunscreen along with an appropriate skin cleansing agent to minimize the comedogenic effects of skin lipids and UV radiation. This article describes a study of the activity of a particular commercial, oil-free topical sunscreen formulationa that contains titanium dioxide and ethyl-hexyl methoxycinnamate for UV protection and phosphatidylcholine and hydrosoluble azelaic acid, to be used in conjunction with a particular oil-free cleansing emulsionb. Both formulations were oil-free so as not to increase the amount of greasy compound on a skin already rich in sebum.
Materials, Methods and Results
Test subjects: Forty-two healthy volunteers were studied, including 24 women and 18 men with an average age of 18±4 years and with a skin color having an Individual Typology Angle (ITA) value greater than 28, which classifies the selected volunteers as normal Caucasians with a skin type of III or IV. Subjects with acne were defined as having a minimum of 15 comedones and 10 inflammatory papules on their face. They participated in the study after filling out and signing an informed consent form.
Exclusion criteria were as follows: presentation with more than five nodules and cysts; use of topical antibiotics, retinoids or benzoyl peroxide in the past 14 days; use of systemic antibiotics in the past 30 days; use of systemic retinoids in the past two years; use of any other topical acne treatments including medical soaps, creams or makeup in the past seven days; and use of corticosteroids, antihistaminics or anti-inflammatory drugs in the past 14 days or systemic corticosteroids in the past 12 weeks. Persons who had been previously involved in any sun test were excluded. Also excluded were persons who presented with sunburn or suntan, scars or active dermal lesions on their back. The volunteers had no previous history of allergy, photoallergy or abnormal responses to sunlight and they had not used tanning beds or tanning products on their back during the previous month. The women were neither pregnant nor lactating. Two participants abandoned the study for unknown reasons.
During the measurements, all volunteers examined by an expert dermatologist were kept in a room with a temperature of 22±2°C and a relative humidity of 50%.
Test substance: Each volunteer was supplied with a tube labeled as cream A (Formula 1a) or cream B (Formula 1b)
together with the cleansing nanoemulsionb. Cream A was the vehicle with the sunscreen actives. Cream B was the vehicle only. During the study neither the volunteers nor the examining dermatologist knew which formula was in the provided tube.
Procedure: This eight-week, randomized, double-blind, vehicle-controlled study was performed in the period from March to April 2006. The subjects were instructed to apply uniformly the test creams (A or B) on the cleansed face (forehead, nose, cheeks and chin) at least twice a day for the duration of the study period and were not allowed to use other skin care products. In addition, subjects were not allowed to use other acne treatments during the study. Finally, subjects were told not to apply the cream on the day of the evaluation and not to wash their face during the four hours prior to the evaluation.
Measurement of skin hydration and superficial skin lipids: Skin hydration and superficial skin lipids were evaluated instrumentallyc using a methodology described by Cardillo et al.14–15 This computerized instrument has a separate probe for each of these test parameters. The probes collect up to 15 separate readings over a 25-sec sampling period.
On the days of laboratory evaluations, the skin was cleansed in the morning before measurements were taken and then left undisturbed until after these evaluations were completed. The individual readings were taken between the nose and cheek and were automatically averaged together. The resulting mean value was documented after standardization for environmental conditions, at a relative humidity of 25% and a temperature of 22°C.
The probe employed for the measurement of skin hydration specifically assesses the total capacitance of the epidermis. The values are expressed in arbitrary units by the computer-controlled system. All skin hydration measurements were taken under standardized conditions.16
The probe employed for the measurement of superficial skin lipids employs a 1 cm2 frosted plastic foil surface that is brought into contact with the surface of the skin. Upon contact with the lipids on the surface of the skin, this frosted foil becomes transparent in direct proportion to the amount of lipids present on the skin. The change in the light transmission of the foil is automatically recorded by the instrument and converted to milligrams of lipid per square centimeter of skin surface.
The mean readings obtained are shown in Figure 1 and Table 1.
Free fatty acids and triglycerides: The special and protected plastic foil was applied on the 5 facial areas with gentle pressure by 20 strokes of a gloved finger and carefully removed. Stratum corneum lipids were extracted from the frosted plastic foil using chloroform:methanol (2:1) for 2 hr at room temperature, the solvent was dried under nitrogen, and the lipids were redissolved in chloroform. Lipid fractions were chromatographically separated on 0.25-mm-thick layers of silica gel into their individual lipid classes and were successively separated by plastic foil contaminants, using solid phase extraction columns, according to previous experiments.5 All lipid fractions, redissolved in chloroform, were stored at –20°C until required.
The isolated triglycerides and free fatty acids were then quantified separately in order to determine the ratio of free fatty acids to triglycerides, reported in Figure 2 as mean values.
Propionibacterium acnes counts: In accordance with previous studies,5P. acnes was evaluated using the method17 of Williamson and Kligman according to McFinley et al.18 Samples were taken on the first day for a baseline value and after 2, 4, 6 and 8 weeks of treatment.
Before the sample was taken, the face of the subjects was cleaned by using a sterile gauze saturated with a 0.1% sterile solution of a detergentd, followed by a further cleaning with sterile distilled water and finally rubbed with a gauze saturated with hexane for 30 sec. The cleansed skin was protected with a porous sterile plastic gauze to maintain normal evaporative activity. After 1 hr, a cylinder of sterile glass with hollow base was applied to each area and 1mL of sterile solution of 0.1% of the detergent in a pH 7.9 phosphate buffer was introduced. After the area was cleaned with a nonsticke spatula for 1 min, two successive samples of liquid were taken. The samples thus obtained were diluted a number of times with a solution of 0.005% detergent immediately set in culture with a solution of 0.1% polysorbate 80f and incubated anaerobically for seven days. The obtained mean results are reported in Figure 3.
UVB protection: UVB protection was evaluated 24 hr after irradiation by means of the Colipa method, as previously reported,18 on the backs of 20 selected volunteers of the study group in a predetermined area between the scapula line and the waist.
A solar simulator was used and its output irradiance was controlled by means of a calibrated radiometer. For each subject, the unprotected minimal erythema dose (MEDu) was previously and colorimetrically measured (color matching) instrumentallyg without resorting to UV exposure.
The obtained mean SPFs obtained for the active sunscreen, the sunscreen vehicle and a standard commercial product were 23±2.42, 5±0.97 and 18±2.0, respectively. At 95% confidence level, cream A was significantly different from cream B and the standard (P < 0.05).
UVA protection: UVA protection was evaluated on the backs of the other 20 subjects of the study group, as previously reported.19 The unprotected minimal pigmenting doses (MPDu) and protected minimal pigmenting doses (MPDp) were determined on the same day at the beginning of the study, 15 and 30 min after the application of the tested product, while maintaining the subjects in the prone position.
The source of irradiation used was a solar simulatorh fitted with appropriate optical cut-off filtersk essential for eliminating wavelengths below 320 nm (UVB)and above 400 nm (visible light and infrared). These filters yield only the UVA spectrum, in accordance with the authors’ previous experience19 and the European Union’s memo on sunscreens.20
The determination of the test area exposed to the UVA flux in a geometric progression of 25% (1, 25, ...) was the same for the unprotected and protected area.
The MPDu were taken every 6 min over 3 hr of exposition and MPDp were determined instrumentallyg 30 min after the test product was applied onto the area that had been previously cleansed with a pad soaked with distilled water.
The UVA-PF (protection factor) was calculated as the ratio between the minimum UVA dose needed to induce the minimal darkening effect on the skin protected by the test product (MPDp) and the minimum UVA dose needed to induce the minimal darkening effect on the unprotected skin (MPDu).
Each individual result for both parameters and all the colorimetric information relating to the involved subjects were validated by an expert dermatologist at the time of the test. The UVA-PFs obtained for the active sunscreen, the sunscreen vehicle and the standard commercial product were 8.5±1.6, 2.1±0.9, and 6.8±1.3, respectively. At a 95% confidence level, cream A was significantly different from cream B (P < 0.005) and the standard (P < 0.05).
Determination of skin’s enzymatic production: It is common knowledge that higher propionibacteria growth entails an increased production of lipase and hyaluronidase.21 Therefore, a quantitative evaluation of the presence of these two enzymes on the skin of the volunteers was conducted at the beginning of the treatment period, i.e., the baseline value, and throughout the treatment periodweek 2, 4, 6 and 8 according to Cove et al.21 The obtained results are shown in Figure 4.
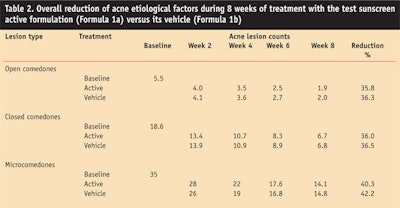
Clinical examinations: Clinical examinations were performed on the first day or baseline, throughout the treatment period of weeks 2, 4 and 6, and at the end of treatment, i.e., week 8. Clinical evaluations included individual lesion counts of open and closed comedones, papules and pustules, and were quantified by calculating them separately by means of a transparent millimeter grid of cellophane, according to Morganti et al.5
The results were scored according to the following scale of percentage reductions in lesion counts: very good (>75% reduction), good (50–74%), moderate (25–49%) and poor (<25%); unchanged and worsened were also scored. The obtained results are shown in Figure 5 and Table 2.
Statistical evaluations: Statistical evaluations of the test results were run on softwarem able to conduct both parametric and nonparametric analyses such as one-way, two-way and repeated measures ANOVA techniques; post ANOVA analyses such as Tukey analyses; Pearson analyses; and least squares linear fitting of test results. All statistical evaluations were conducted as two-tailed analyses at a minimum of a 95% confidence interval (P < 0.05) to determine statistically significant differences in the results.
Discussion
The results indicate that it is possible to formulate sunscreen products that maintain a broad-spectrum photoprotective efficacy against both UVB and UVA while at the same time providing activity against some key factors of the pathogenesis of acne. These key factors include an increased production of free fatty acids and increased number of P. acnes colonies. The results obtained with the use of this cosmetic formulation seem due to the presence in the vehicle of hydrosoluble azelaic acid with its strong antibacterial potency22 and phosphatidylcholine from soybeans, which is effective in normalizing the reduced linoleic content in the sebum.23 As Figures 1–5 and Table 1 show, the studied products including vehicle and active display an interesting activity on acne-prone skin. Moreover, it was observed that the active cream also provides high protection against UVB and UVA rays because of the sunscreen compounds used.
Figure 1b and Figure 2 show that both the vehicle and the active were able to decrease superficial skin lipids i.e., sebum outflowing, as well as the skin’s ratio of free fatty acids to triglycerides, restoring the normal and healthy condition of an acne-prone skin. It should be remembered that in acne skin there is an increase of free fatty acids resulting from hydrolysis of triglycerides due to the activity of the lipase, protease, hyaluronidase and other enzymes produced from propionibacteria. Moreover the vehicle, enriched with phosphatidylcholine and azelaic acid, gives to the photoprotective sunscreen product a high efficiency against the main causes of acne.
In fact, many scientists have demon-strated that sebum production is the main driving force in the development of acne; meanwhile, free fatty acids may contribute to comedogenicity.24
Both the vehicle and the active enabled a continuous decrease in propionibacteria (Figure 3) and an improvement in the overall clinical results (Figure 5), in accordance with the authors’ previous research studies also demonstrating the influence of the adopted cleansing methodology.5–7
Both the vehicle and the active also decreased the typical enzymatic activity involved in the irritation and destruction of the follicular wall25 observed in acne-prone and oily skin (Figure 4). As matter of fact, the activity of both hyaluronidase and lipase declined constantly throughout the study period. This further shows the ability of phosphatydylcholine and azelaic acid to reduce the quantity and quality of sebum fatty acid components when used in well-calibrated amounts.
Finally, the obtained data showed the effect of the active product in protecting against both UVB and UVA rays.
Conclusion
Therefore, it seems possible that phosphatydylcholine and azelaic acid can be used to formulate sunscreen products capable of protecting against UV rays (both UVA and UVB) while at the same time improving the acne condition. This data is useful for formulating products targeted to oily and acne-prone skin. It may also be useful to help reduce the follicular eruptions generally caused by UV radiation, mainly UVA, and resulting from PUVA therapy.26–27
Moreover, because the described nanoemulsion normalizes excessive skin oiliness and the consequent follicular hyperkeratinization, it may be indicated for the topical management of oily skin and in mild to moderate forms of comedonic and papulopustular-type acne during the summer period or in sunny countries.
Acknowledgment: The authors thank Mavi Sud and the dermatology departments of the universities of Messina and Naples for supporting this study.
References
1. ChC Zouboulis, Sebaceous glands, acne and related disorders, Dermatology 196(1) 1–188 (1998)
2. A Kligman and AG Katz, Pathogenesis of acne vulgaris comedogenic properties of human sebum in external ear canal of the rabbit, Arch Dermatol 98 53–55 (1968)
3. P Kanaar, Follicular-keratogenic properties of fatty acids in the external ear canal of the rabbit, Arch Dermatol 98 53–55 (1971)
4. P Morganti et al, Effect of phosphatidylcholine linoleic acid-rich and glycolic acid in acne vulgaris, J Appl Cosmetol 15 21–32 (1997)
5. P Morganti, A Agostini, C Bruno and G Fabrizi, Role of topical glycolic acid and phosphatidylcholine linoleic acid-rich in the pathogenesis of acne, J Appl Cosmetol 15 33–41 (1997)
6. G Fabrizi, SD Randazzo, A Cardillo, L Tiberi and P Morganti, Safety and efficacy of a lamellar phosphatidylcholine emulsion to treat mild-to-moderate inflammatory acne, SÖFW Journal 125 12–15 (1998)
7. P Morganti, G Fabrizi and Xin Zhong Feng, A new delivery system to improve acne therapy, Eurocosmetics 10(5) 33–37 (2002)
8. M Allem and P Lopresti, Acne vulgaris aggravated by sunlight, Cutis 26 254–328 (1980)
9. T Fitzpatrick, Sunlight and Man: Normal and Abnormal Photobiologic Responses, Tokyo: University of Tokyo Press (1980) pp 353–374
10. M Gloor and A Klarenfeld, Effect of ultraviolet light therapy given over a period of several weeks on the composition of the skin surface lipids, Dermatologica 154 5–13 (1997)
11. DC Steinberg, Regulations of sunscreens worldwide, In Sunscreens: Regulations and Commercial Development, 3rd edn, NA Shaath, ed, New York: Marcel Dekker (2005) pp 173–198
12. K Klein and C Palefsky, Formulating sunscreen products, In Sunscreens: Regulations and Commercial Development, 3rd edn, NA Shaath, ed, New York: Marcel Dekker (2005) pp 353–383
13. MAVI data on file (2005)
14. A Cardillo and P Morganti, Fast and non-invasive method for assessing skin hydration, J Appl Cosmetol 12 11–16 (1988)
15. G Fabrizi, A Lanzone and F Cucinelli, Metodologie non invasive di valutazione cutanea: 3C System—the check-up, In Metodologie Diagnostiche, Preservative Correttive, CA Bartoletti, ed, Rome: Editrice Salus Internazionale (1998) pp 703–716
16. J Pinnagoda, Standardization of measurements, In Bioengineering and the Skin: Water and Stratum Corneum, P Elsner, E Berardesca and H Maibach, eds, Boca Raton: CRC Press (1994) pp 59–65
17. P Williamson and AM Kligman, A new method for the quantitative investigation of cutaneous bacteria, J Invest Dermatol 45 498–501 (1965)
18. K McFilley, G Welster and J Leyden, Regional variations in cutaneous propioniobacteria, J Appl Env Micro 45 998–1000 (1965)
19. P Morganti and G Fabrizi, New data on skin photoprotection, Int J Cosmet Sci 22 305–312 (2000)
20. ec.europa.en/enterprise/cosmetics/sunscreens/index en.htm
21. JH Cove, WJ Cunliffe and KT Holland, Acne vulgaris: Is the bacterial population significant? Br J Dermatol 102 277–285 (1980)
22. H Gollnick and M Schramm, Topical drug treatment in acne, In Dermatology: Sebaceous Glands, Acne and Related Disorders, ChC Zouboulis, ed, Basel: Karger (1998) pp 119–125
23. M Ghyczy, HP Nissen and H Bilz, The treatment of acne vulgaris by phosphatidylcholine from soy beans, with a high content of linoleic acid, J Appl Cosmetol 14 137–145 (1996)
24. DT Downing et al, Essential fatty acids and acne, J Am Acad Dermatol 14 221–225 (1986)
25. H Akamatsu and T Horio, The possible role of reactive oxygen species by neutrophils in mediating acne inflammation. In Dermatology: Sebaceous Glands, Acne and Related Disorders, ChC Zouboulis, ed, Basel: Karger (1998) pp 82–85
26. N Hjorth, KE Sjolin, B Sylvest and K Thomsen, Acne aestivalis-mallorca acne, Acta Derm Venereol (Stockh) 52 61–63 (1972)
27. EB Nielson and J Thorman, Acne-like eruptions induced by PUVA treatment, Acta Derm Venereol (Stockh) 58 374–375 (1978)