A combination of factors affects skin functionality and appearance. Internal factors modulate the expression of key genes involved in skin homeostasis. Such intrinsic aging is genetically regulated and follows a chronological clock inside of cells, whereas environmental factors are responsible for actinic aging. Together, these factors target important metabolic pathways in skin cells that trigger the signs of aging, such as skin roughness and loss of biomechanical properties. At the molecular level, collagen synthesis is reduced in aging skin cells and cells damaged by UV radiation.1 The expression of collagen-degrading matrix metalloproteinases (MMPs) is upregulated in aged cells and cells exposed to UV, infrared radiation or excessive heat.2, 3 Also, reactive oxygen species are implicated in the deleterious effects of aging.1, 4 In an effort to develop a natural active to address these skin aging issues, the efficacy of Hesperomeles heterophylla leaf extract was studied as a novel natural source for anti-aging actives that provide multiple skin benefits, as described in this paper.
H. heterophylla Habitat
For the past five million years, the Andes Mountains in South America lifted, eventually settling and creating the Andean forests—and on the top of these, the páramo and subpáramo ecosystems.5, 6 These ecosystems are located discontinuously from altitudes of approximately 2,500 m to the line of perpetual snow, at about 5,000 m. The lower density of the atmospheric layer at these high altitudes allows for greater UV radiation intensities as well as greater dissipation of light energy. In relation, one of the most important research fields in Latin America is the scientific validation of traditional knowledge for the use of biodiversity and its applications.7
Based on this information, the species H. heterophylla Hook was selected to evaluate its effects as an anti-aging active. Hesperomeles is a genus of flowering plants belonging to the family Rosaceae. H. heterophylla grows in the subpáramo and páramo ecosystems at 2,500–3,500 m and has adaptations that allow it to survive in this fairly hostile environment. For example, it has adapted to daily seasonality, cold, low air pressure and low water availability.
The shrub size is small, to keep out the cold and wind, and the plant has hard leaves that prevent water loss by evapotranspiration. Among the most important traits of genus Hesperomeles is its anthocyanin content, which is of interest to the foods industry and is useful as a dye and antioxidant.8 It is also traditionally known for its anti-inflammatory activity and has been used to treat kidney problems. Further, its wood is used to make handicrafts, cabinets and charcoal. Lastly, its small red fruits can be consumed directly or used to make jams and drinks.6 In certain genuses polyphenols have been found, although they have not been studied for biological activity. Thus, the goal of the present work was to characterize the H. heterophylla Hook leaf extract and study its in vitro and in vivo efficacy.
Materials and Methods
As noted, the ability of H. heterophylla to adapt and survive in a hostile environment, together with the accumulation of polyphenols as potential protection compounds, led the authors to investigate this material’s potential antioxidant activity, ability to promote the expression of structural extra-cellular matrix (ECM) components, and ability to inhibit the proteolytic enzyme MMP1. Clinical benefits for the skin microrelief and elasticity were also investigated.
Extract and phytochemical analyses: The extract was prepared starting from the leaves of native H. heterophylla Hook scrub since they showed lower cytotoxicity than the fruits. Branches were collected near the town of Ubaté, Colombia. The specimens were identified by the vernacular name and later validateda. The leaves were then washed with sterile water and dried at 40°C. Using a grinder, 600 g of dried leaves were finely cut.
The extraction process was performed by a cold percolation method with three solvents: petroleum ether, chloroform and ethanol. Each time, the solvent was removed in a rotary vacuum evaporator. The ethanolic extract was resuspended in 25 mL of ethanol: water (1:7). This content was transferred to a separator funnel to produce a liquid-liquid extraction with two portions of 15 mL ethyl acetate. The organic phase was stored in an amber vial. The aqueous fraction was extracted with two portions of n-butanol, 15 mL each. Aqueous and butanolic fractions were obtained and analyzed by thin layer chromatography (TLC). Throughout the study, the ethanolic extract was used by dissolving it in appropriate solvents or vehicles, depending upon the assay to be performed.
Total phenolic content (TPC): TPC was measured by the Folin-Ciocalteu method based on the formation of blue-colored products from redox reactions.9 Absorbance was measured at 750 nmb. The TPC was expressed as gallic acid equivalents (GAE) in mg/g extract, obtained from the standard curve of gallic acid solutions established by plotting concentration versus absorbance (see Figure 1). Tests were carried out in triplicate.
Free radical-scavenging activity: To determine free radical scavenging activity, an in vitro, cell-free chemically based DPPH scavenging test10 was performed using either H. heterophylla leaf extract at different concentrations, or the synthetic antioxidant 2,6-bis(1,1-dimethylethyl)-4-methylphenol (BHT) as a positive control. The absorbance was read at 517 nmb since, due to the presence of an odd electron, DPPH gives a strong absorption maximum at this wavelength. Inhibition of the free radical DPPH or I% was calculated as: I% = (Ablank-Asample/Ablank) x 100, where Ablank refers to the absorbance of the control reaction (containing all reagents except the extract), and Asample is the absorbance of the extract. Extract concentration providing 50% inhibition (IC50) was calculated from the graph plotting inhibition percentage against extract concentration. Tests were carried out in triplicate.
Cell viability: The viability of normal human epidermal keratinocytes (NHEK)c and normal human dermal fibroblasts (NHDF)c treated with H. heterophylla leaf extract was determined by MTT assay,11 measuring absorbance at 570 nmb—the wavelength at which the blue-colored formed formazan crystal concentration can be determined, post reduction of tetrazolium dye (MTT) via NAD(P)H-dependent cellular oxidoreductase activity. This reflects the number of viable skin cells.
Oxidative stress: NHEK cells were incubated for 45 min in the presence or absence of the H. heterophylla leaf extract at different concentrations, or the positive control butylated hydroxyanisole (BHA, 100 µM); and in the presence of the specific fluorescent probe dihydrorhodamine (dHR) to evaluate hydrogen peroxide (H2O2) production. After incubation, cells were irradiated or not (control) at UVB 180 mJ/cm2 and UVA 2.5 mJ/cm2, and incubated for 30 min before flow cytometry analysisd. The fluorescent intensity of the dHR probe is proportional to H2O2 production expressed in fluorescence arbitrary units (AU).
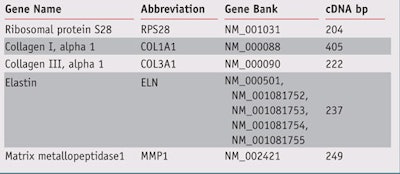
Gene expression: NHDF were incubated in the presence or absence of H. heterophylla leaf extract or the reference compound TGF-β (10 ng/mL) for 24 hr. The cells were then washed and stored at -80°C until the next step. Gene expression was analyzed using the RT-qPCR methode. Primers sets were used to amplify the fragment corresponding to selected genes (see Table 1). Results were normalized using the ribosomal protein S28 gene (RPS28) as a marker.
The incorporation of fluorescence in amplified DNA was measured during the PCR cycles. This resulted in a “fluorescence intensity versus PCR cycle plot,” allowing the evaluation of a relative expression (RE) value for each gene. The value selected for RE calculations is the output point of the fluorescence curve. For a considered gene, the smaller the amount of mRNA, the greater the number of cycles required. The RE value was expressed in arbitrary units (AU) according to the formula: (1 / 2 number of cycles) x 106.
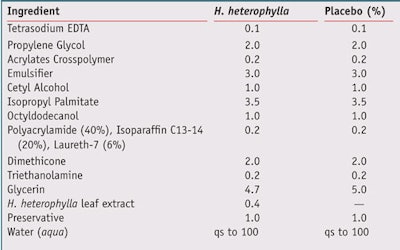
Formulation: An oil-in-water formula containing 0.35% w/w H. heterophylla leaf extract also was evaluated in vivo and compared with the same formula without the extract (see Table 2). The extract was first dissolved in glycerin before being incorporated into the formulation.
Efficacy in vivo: Forty volunteers ages 35–45 were enrolled in a trial using a randomized protocol to evaluate the efficacy of the formulations in Table 2. Each volunteer applied approximately 0.8 g of the formula to their clean faces twice daily for a period of 28 days. Measurements were performed at baseline prior to application of the formulations and after 14 and 28 days. Variations in skin roughness parameters were measured using an optical 3-D in vivo skin measuring systemf based on stripe projection.12 Variations of skin bio- mechanical parameters were determined based on the suction chamber principleg; i.e., skin movement into the probe during negative pressure and its movement out when pressure is released can be displayed as a time-strain curve, allowing the determination of parameters describing the skin’s extension and relaxation behavior.13, 14 Statistical analyses were performed using the student’s t-test for paired and unpaired data. All experimental conditions were performed in triplicate.
Results and Discussion
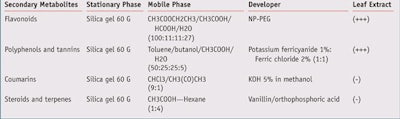
Phytochemical analyses: TLC analyses showed the H. heterophylla leaf extract to be rich in polyphenols and flavonoids (see Table 3); the total phenolic content (TPC) and antioxidant properties of the extract are described in Table 4.
Antioxidant properties: Free radicals form in the human body during normal cellular metabolism and following exposure to UV light, gamma radiation, cigarette smoke and other environmental pollutants.15 To calculate the radical scavenging power of the H. heterophylla leaf extract, as noted, the DPPH assay was performed. DPPH is stable at room temperature and produces a violet solution in ethanol. In the presence of antioxidant compounds, DPPH is reduced, producing a colorless ethanol solution. The extract exhibited extremely significant (***: p < 0.001), dose-dependent activity with 95% scavenging capacity at 50 µg/mL (see Figure 2). This was three times more active (IC50 at 8.69 ± 0.71 µg/mL) than BHT (IC50 at 25.79 ± 1.42 µg/mL) tested in parallel under the same concentrations (see Table 4).
Disturbances in the normal redox state of cells can cause toxic effects through the production of peroxides and free radicals, which damage all components of the cell.15–17 One of the strongest oxidant species is hydrogen peroxide (H2O2). Organisms naturally produce H2O2 as a by-product of oxidative metabolism. Consequently, all aerobic organisms possess enzymes that catalytically decompose H2O2 to water and oxygen. Since aging is associated with oxidative stress16, 17 and it has been reported that flavonoids show pharmacological effects as antioxidants, these beneficial properties were verified for the H. heterophylla leaf extract by means of a UV-induced H2O2 production “in cell” assay. Irradiation of keratinocytes with 180 mJ/cm2 of UVB clearly induced peroxide production of the untreated cells, showing the cell response to the oxidative stress.
Pretreatment with the synthetic antioxidant BHA at 100 µM significantly protected cells from UV-induced H2O2 production, with reduction of 28% compared with the untreated control (**: p 0.001–0.01). H. heterophylla leaf extract protected keratinocytes in a concentration-dependent manner from UV-induced H2O2 production (see Figure 3); 45 min of pretreatment with the extract at 37 µg/mL resulted in a significant 63% reduction of UV-induced H2O2 production (*** : p < 0.001), compared with the untreated control.
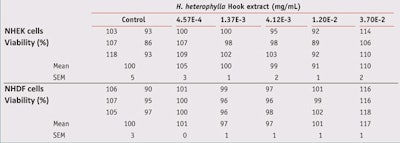
Cell viability: Before H2O2 production and gene expression assays, the adequate dose of H. heterophylla leaf extract was determined for use in the MTT assay,11 which is quantitative in that it measures the metabolic activity of viable cells based on the ability of mitochondrial dehydrogenases of the cells to reduce 3-(4,5-dimety-2 thiazoyl)-2,5-dipheny-2H-tetrazolium bromide (MTT), yellow and hydrophilic, to dark blue hydrophobic compounds (formazan). The H. heterophylla leaf extract did not decrease normal skin human keratinocyte (NHEK) or fibroblast (NHDF) viability in the concentrations used (see Table 5).
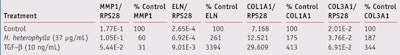
Gene expression: Alterations in collagen and elastin, which form the extracellular matrix (ECM), are responsible for the clinical manifestations of skin aging, including wrinkles, sagging and laxity.16 The fibrillar collagens types I and III provide structure whereas elastin forms elastic fibers that give skin firmness and elasticity.17 The atrophy of collagen fibers in skin aging occurs from reduced synthesis and the increased expression of their degradative enzymes, especially matrix metalloproteinase 1 (MMP-1).2 Therefore, the effects of the H. heterophylla leaf extract on relative gene expression of ECM components in NHDF cell culture were evaluated after 24 hr of treatment.
The extract at 37 µg/mL inhibited the expression of MMP-1; this concentration range of MMP-1 expression inhibition correlates well with the obtained inhibition exerted in vitro by this extract on Clostridium histolyticum collagenase enzymatic activity (IC50 27.54 µg/mL), observed in a previous study.18 On the other hand, the extract strongly stimulated the expression of ELN, COL1A1 and COL3A1 (see Figure 4). The TGF-β treatment (10 ng/mL) clearly stimulated the expression of COL1A1, COL3A1 and ELN, and inhibited MMP-1, validating the assay (see Table 6).
Efficacy in vivo: Improved skin firmness and elasticity, and a decrease in wrinkles are major targets for anti-aging claims in skin care products. The alteration of these functions during skin aging is due to the degradation of collagen and elastic tissue.16 Since the extract inhibited MMP-1 and increased expression of collagen and elastin, it was tested in vivo in a formulation containing 0.35% H. heterophylla leaf extract. Variations of the roughness parameter Rz, i.e. maximum roughness average,12 also were measured (see Figure 5).
The formulation incorporating the extract induced an average decrease in wrinkle depth of 11% by day 14, and a significant decrease (* p < 0.05) of 13% by day 28. This effect was measured in 68% and 75% of the volunteers, respectively. The placebo used in parallel induced a small effect with no relevant variation of Rz. Figure 6 shows a topographic image of the skin surface, comparing the appearance of the crow’s-feet area on the first day with the same area on day 28. In this image, the blue areas are the deeper areas; note that the blue color disappears after 28 days of formulation use.
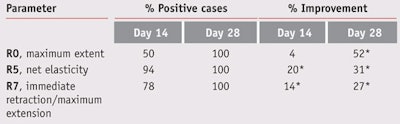
Variations of parameters describing skin extension and relaxation behavior also were evaluated. The maximum extent to which the skin could be extended (R0) decreased over the course of the treatment. At 28 days, volunteers’ skin could be extended the least (see Figure 7), and the differences both with the placebo and with the first day were significant (* p < 0.05). Net elasticity (R5), i.e., the ratio of immediate retraction to immediate extension, increased to its greatest extent at the end of the study (see Figure 8a). The ratio of immediate retraction to maximum extension (R7) increased over the duration of the study (see Figure 8b). For parameters R5 and R7, the differences between the formulation with the placebo and with the first day were significant (* p < 0.05) by days 14 and 28 (see Table 7).
Conclusions
H. heterophylla Hook leaf extract was shown in this study to exhibit antioxidant properties due to the presence of high levels of polyphenols and flavonoids. It significantly reduces the UV-induced H2O2 production of human epidermal keratinocytes, indicating its potential to help cells to withstand UV stress. In addition, it demonstrated dual protective effects on the ECM via the inhibition of the transcript expression of ECM proteolytic enzyme MMP-1, and the stimulation of the structural ECM components COL1A1, COL3A1 and ELN. Thus, the extract is a potential stimulator of ECM synthesis.
In vivo, promising results were obtained, as the ingredient decreased wrinkle depth and improved skin extension and relaxation behaviors. These results highlight the use of this leaf extract, from a scrub of the Latin America biodiversity, as an alternative biological source of actives with proven skin benefits, for the development of multifunctional skin care products.
References
Send e-mail to [email protected].
1. TM Callaghan and KP Wilhelm, A review of ageing and an examination of clinical methods in the assessment of ageing skin. Part I: Cellular and molecular perspectives of skin ageing, Int J Cosmet Sci 30 (5) 313–322 (2008)
2. T Quan, Z Qin, W Xia, Y Shao, JJ Voorhess and GJ Fisher, Matrix-degrading metalloproteinases in photoaging, J Invest Dermatol Symp Proc 14 (1) 20–24 (2009)
3. S Cho, MH Shin, YK Kim, JE Seo, YM Lee, CH Park and JH Chung, Effects of infrared radiation and heat on human skin aging in vivo, J Invest Dermatol Symp Proc 14 (1) 15–19 (2009)
4. DR Bickers and M Athar, Oxidative stress in the pathogenesis of skin disease, J Invest Dermatol 126 (12) 2565–2575 (2006)
5. M Morales et al, Atlas of Colombia Páramos, Research Institute for Bioresources Alexander von Humboldt, Bogotá, Colombia (2007)
6. JL Toro, GL Vanegas, and C Peláez, Flora of the Páramos and Andean Forest of northwestern of Antioquia, Corantioquia Multimpresos Ltda, Colombia (2002)
7. W Roca, C Espinoza, A Panta, and G Trujillo, Trends in the development of biotechnology and institutional capacities for the use of biodiversity in the countries of the Andean Community, report prepared for the Economic Commission for Latin America and the Caribbean (ECLAC) and the Andean Development Corporation (CAF) (2004)
8. C Garzón, Basic food science and phytochemical analyzes of priority species within the framework of the project: Sustainable use of the Capital District and region plant resources, unpublished technical report, Jose Celestino Mutis Botanical Garden, Scientific Office, Bogotá, Colombia (2006)
9. VL Singleton and JA Rossi, Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents, Am J Enol Viticulture 16 144–158 (1965)
10. MS Blois, Antioxidant determinations by the use of a stable free radical, Nature 29 1199–1200 (1958)
11. T Mosmann, Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays, J Immunol Methods 16 55–63 (1983)
12. Operating Manual Optical 3-D in vivo Skin Measuring System based on Stripe Projection by means of Digital Micromirror Devices DMD, Software PRIMOS 5.7, GFMesstechnik GmbH
13. AO Barel, W Courage and P Clarys, Suction chamber method for measurement of skin mechanics: The new digital version of the cutometer, in Handbook of non-invasive methods and the skin, J Serup, G Jemec and G Grove, eds, 2nd edn, CRC Press, BocaRaton, USA (2006) pp 583–592
14. User Manual Cutometer MPA 580, Courage & Khazaka Electronic GmbH, Cologne, Germany
15. M Valko, D Leibfritz, J Moncol, MTD Cronin, M Mazur and J Telser, Free radicals and antioxidants in normal physiological functions and human disease, Internal J Biochem Cell Biol 39 (1) 44–84 (2007)
16. L Rittié and GJ Fisher, UV-light induced signal cascades and skin aging, Ageing Res Rev 1 705–720 (2002)
17. GJ Fisher et al, Mechanisms of photoaging and chronological of skin aging, Arch Dermatol 138 1462–1470 (2002).
18. M Guzmán and Cortázar TM, Inhibition of collagenase and elastase activities in human UVB stimulated fibroblasts, Cosméticos y Tecnología (Latinoamérica) 2 19–23 (2011