In order to avoid microbial contamination and assure the shelf life of cosmetics, active antimicrobial ingredients such as parabens are added to formulations. The success of these widely used preservatives is mostly due to their ease of use and low cost. However, in recent years, as many scientists worldwide have researched the effects of parabens on humans, some studies have suggested that parabens exhibit estrogenic activity, or have found them in human breast tissues.1, 2 This has led to criticism over their presence in skin care products, so in response to consumer fears, formulators have replaced parabens with alternative preservative systems or preservative-free “hurdle” technologies.3, 4 Such technologies often contain natural preservatives or ingredients with antimicrobial properties that are not registered as preservatives. Despite the controversy surrounding parabens, however, they are still present in most cosmetics. Therefore, assessing the skin delivery and penetration of parabens is crucial to estimating their potential risk because they cross the cutaneous barrier and enter the systemic circulation. In this article, percutaneous penetration studies of parabens spanning the past 20 years are reviewed to determine how a number of parameters affect paraben penetration and to assess the risk of various preservative classes as they are formulated into cosmetics.
Despite the controversy surrounding parabens, however, they are still present in most cosmetics. Therefore, assessing the skin delivery and penetration of parabens is crucial to estimating their potential risk because they cross the cutaneous barrier and enter the systemic circulation. In this article, percutaneous penetration studies of parabens spanning the past 20 years are reviewed to determine how a number of parameters affect paraben penetration and to assess the risk of various preservative classes as they are formulated into cosmetics.
Paraben Properties
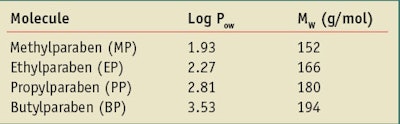
Parabens are a homologous series of hydroxybenzoic acids containing an ester group at the C-4 position, as shown in Figure 1. Popular examples include: methylparaben (MP), ethylparaben (EP), propylparaben (PP), butylparaben (BP), isopropylparaben, isobutylparaben and benzylparaben. Most parabens used in cosmetics include MP, EP, PP and BP and their sodium salts. The hydrophobicity and octanol-water partition coefficient (Pow) of parabens increases with the ester chain length; for example, MP is more water-soluble than BP. Their antimicrobial activity is also related to the chain length of the ester group. Therefore, as the chain length increases, the antimicrobial activity of the paraben increases as the water solubility decreases. These differences in solubility also suggest their use as mixtures, which is often the case.
Parabens with a longer alkyl chain such as PP and BP are the most active against fungi. They also exhibit high activity against Gram-positive bacteria but are considered weak against Gram-negative bacteria, although the amount of paraben dissolved in the water phase determines the preservative’s ability. Generally, microbial replication occurs in a formula’s water phase, so parabens should be soluble in the water phase in order to kill microorganisms.5, 6 Products having a low water content are self-preserved but many cosmetic products such as creams, lotions, shampoos, conditioners and liquid soaps have high water activity and consequently, chemical preservation is necessary.
The European Union and US Food and Drug Administration (FDA) allow a maximum of 0.4% of each paraben, expressed as p-hydroxy benzoic acid (HBA), and a maximum of 0.8% of paraben mixtures in cosmetic products.7 In accordance, an industry survey from 2003 conducted by the Personal Care Products Council, then the Cosmetic, Toiletry and Fragrance Association (CTFA), found that parabens were formulated into cosmetics at 0.0002% to 1%.8 These materials are easy to formulate with because they have no odor, no color effect, are nearly pH neutral and have no effect on the product viscosity. In addition, parabens are stable at high temperatures, so cosmetic products can be safely autoclaved without losing their antimicrobial activity.6
Paraben Penetration
In relation to dermal absorption, recent work by the Organization for Economic Co-operation and Development (OECD) claims that 100% dermal absorption may be assumed if the active molecular weight (MW) of a material is lower than 500 g/mol and the Log Pow is between -1 and +4.9 In the case of parabens, as the ester chain length is increased, the Log Pow is increased in the order of: MP, EP, PP and BP. Table 1 shows the Log Pow and MW paraben values as provided by the OECD. Parabens with a low Log Pow have lower skin retention and permeate better through skin than higher Log Pow parabens. In addition, the permeation rate increases with the paraben’s hydrophilicity.
However, it is also crucial to understand that the vehicle choice is important and, contrary to the OECD approach, must be taken into account for exploiting the penetration results. If one follows the OECD rule alone, referring to Table 1, the result would be 100% paraben penetration in the studies presented in this review. The OECD regulatory approach therefore must be modulated.
The percutaneous penetration studies presented in this review were conducted ex vivo using a Franz cell device with donor and receptor compartments. The results varied from one author to another due to such variables as skin type—i.e., human, minipig, mice, synthetic membrane, etc.; vehicle choice—i.e., solvent, cosmetic formulation; the initial paraben concentration applied to the skin surface; and the barrier condition during the experiment.
Solvent vs. Formulation: Vehicle Choice
Penetration results can vary between a cosmetic formulation and a solvent such as water, acetone or ethanol. The following studies highlight the dual importance of paraben solubility in the solvent and the solvent’s effects on stratum corneum (SC) lipid integrity. In addition, the penetration results from various formulation vehicles are considered.
Solvent effects: Dal Pozzo suggested that parabens with a high Log Pow become less soluble in water and more soluble in the SC, which keeps them from crossing the skin barrier, whereas low Log Pow parabens have less affinity for lipids and often cross the barrier.5 Accordingly, in an aqueous vehicle, parabens with a high Pow (PP and BP) were more soluble in the SC lipids and therefore retained in the upper epidermis layer; they did not penetrate as much as shorter chain parabens.5 However, lipophilic parabens that are soluble in a vehicle such as a w/o emulsion can be retained in the vehicle and will not penetrate the SC. Dissolved in water, parabens were found to follow a negative correlation between their Log Pow and skin permeation; low Log Pow parabens penetrated deeper.10
Caon studied the effects of ethanol concentration on paraben skin permeation. A solution of ethanol/phosphate buffer (20/80 or 50/50) with a combination of parabens was dropped on pig ear skin for 6 hr and 7.5 hr, respectively. Lipophilic parabens were found to have a lower permeation flux due to the strong interactions with the SC lipids and were retained in the upper epidermis. At 20%, the presence of ethanol led to a higher paraben retention in the epidermis than the dermis whereas at 50% ethanol, an increase in paraben permeation was observed, which promoted greater accumulation in the dermis.11
Regarding ethanol and acetone as vehicles, Cross did not observe differences in paraben penetration between them.12 In the presence of ethanol, Merle reported that the SC lipid cement could be destabilized ex vivo with the emergence of pores.13 This study revealed that paraben permeation through the skin decreased with an increase of the partition coefficient. Caon and Dal Pozzo also suggested that lipophilic parabens are less soluble in hydrophilic vehicles but more soluble in SC lipids, and that they are retained in the SC.10, 11 The dermis is more hydrophilic, thus parabens have less difficulty passing through it and the aqueous vehicle and SC lipids act as a partition coefficient.
Moreover, the solvent can have an impact on paraben metabolism. For example, MP metabolization in HBA, a less toxic metabolite, is inhibited in the presence of ethanol.14 MP percutaneous penetration in the presence of ethanol was studied on Yucatan micropig skin by Oh,14 who measured MP, EP and HBA concentrations in the receptor phase in the presence or absence of ethanol with phosphate-buffered sulfate (PBS) in the donor compartment. With no ethanol, MP and HBA concentration increased linearly with time in the receptor compartment whereas MP and HBA flux concentration decreased as ethanol concentration increased in the donor compartment. With 10% ethanol in the donor compartment, HBA flux decreased and a new EP flux appeared. The researchers suggest that ethanol enhanced MP transesterification into EP and inhibited MP bioconversion into HBA.14
This data highlights the dual importance of paraben solubility in the solvent, and the solvent effect on the SC lipid integrity.
Formula effects: Cross compared the influence of an ointment or solvents on paraben penetration. MP total penetration and flux were found to be significantly smaller after application of an ointment, compared with acetone or ethanol as the vehicle.12
Dal Pozzo studied emulsion influence and found that parabens in an o/w emulsion penetrated more than parabens in a w/o emulsion. In this study, contact of the o/w emulsion with the skin surface and water phase—i.e., the phase containing the parabens—increased the affinity of lipophilic parabens for the apolar SC. In a w/o emulsion, the oily phase is in contact with the SC, thus affording lipophilic parabens more affinity for the oily phase rather than the SC. Thus, paraben skin permeation from o/w emulsions was higher than from aqueous solutions. It should be noted, however, that the presence of surfactants either increased the paraben concentration in the water phase or damaged the SC.10
Esposito compared the diffusion of parabens from an aqueous solution, an o/w emulsion, a w/o emulsion and two hydrophilic gels: a carboxyvinyl polymer gela and an acrylates/C10–30 alkyl acrylate crosspolymerb.15 All formulations contained 0.05% MP, EP and PP, and the permeation was tested through a synthetic membrane. The diffusion coefficient (flux/concentration) was much higher in the aqueous form, compared with the viscous forms. In addition, PP permeation from water, w/o emulsion and o/w emulsion was less than MP and EP.
Paraben diffusion coefficients from the carbomer gel were seven times higher than from the acrylates/C10–30 alkyl acrylate crosspolymer gel, which is because the acrylate with a C10–30 alkyl chain dissolves the parabens better in the gel matrix. Parabens are then more retained in the formulation, compared with the carbomer gel. This result suggests that the use of acrylates/C10–30 alkyl acrylate crosspolymer could reduce paraben diffusion through the skin.
EP and PP diffusion coefficients were less in the o/w emulsion, compared with the w/o emulsion, whereas the MP coefficient diffusion was higher in the o/w emulsion. MP is mostly dissolved in the aqueous phase, which diffuses faster in the receptor compartment, whereas EP and PP are less soluble in water and may tend to be dissolved in the oil phase. These results are not comparable to Dal Pozzo’s experiments, as a synthetic membrane was used rather than real skin.15
The influence of cyclodextrin on paraben penetration was examined in vitro on hairless mouse skin by Tanaka et al. using either 2-hydroxypropyl-β-cyclodextrin (HP-β-CyD) or the nonionic surfactant polyoxyethylene hydrogenated castor oil (HCO-60). The cutaneous permeation rate of MP was found to significantly decrease with the addition of cyclodextrin whereas its solubility increased linearly as a function of HP-β-CyD concentration. The decrease was greater with cyclodextrin, compared with the surfactant. The researchers also studied the bioconversion of MP into HBA and found that the HBA concentration increased and MP concentration decreased significantly in the presence of cyclodextrin. Further, in vitro, cyclodextrin significantly reduced MP absorption and promoted its bioconversion into HBA.16
Kitagawa studied the permeability of a liposome suspension of MP, EP, PP and BP in PBS through pig skin17 and found that the permeability increased with the increase of Log Pow. The researcher also observed a change in permeability when the SC lipids were depleted. This lipid depletion significantly increased the permeability for hydrophilic parabens in the order of: MP, EP and PP. Kitagawa therefore proposed that parabens penetrate via non-SC lamella.17
These studies show that cosmetic formulation influences mainly paraben penetration through skin, so it is crucial for formulators to decide which paraben to add based on the function of the formulation. It is also important to examine whether any ingredient(s) in the formulation enhance or slow paraben penetration. Kitawaga’s results were the opposite of the other penetration studies, which underlines the fact that ex vivo absorption studies are difficult to generalize and shows that every formulation must be tested.
Changing Paraben Solubility
Some ingredients decrease or increase paraben permeation by changing its solubility in a vehicle while other ingredients fluidize SC lipids and, by disrupting the barrier, increase the permeability of parabens. For instance, the transdermal permeation of MP, EP, PP and BP was tested in water or nicotinamide on rabbit ear skin by Nicoli.18 With nicotinamide in the donor compartment, transdermal penetration significantly increased for PP and BP while it was unchanged for MP and significantly decreased for EP.18 The effects of glycol also were tested, and when propylene glycol or poly-ethylene glycol 400 was added to water in the donor compartment, permeation decreased because these molecules increase paraben solubility in water.
In addition, glycols increased lipophilic paraben solubility in the vehicle, compared with the SC, therefore lipophilic parabens are retained in the solution and penetrate the SC less.10 Similarly, when parabens were dissolved in a lipophilic vehicle such as liquid paraffin, the permeation tended to decrease for parabens with a high Log Pow. In relation, Romonchuk studied the effect of 4-cyanophenol (CP) on the permeability of MP into the human epidermis. With the presence of CP in a saturated solution of MP, MP permeation rates and SC uptake significantly increased by increasing MP solubility in the SC.19
The effect on SC fluidization was observed by Kitawaga with the addition of 1% l-menthol in 15% ethanol, which significantly increased the permeation of MP in a liposome suspension. It had no effect on PP and EP, although it decreased BP penetration. The same tendency, but weaker, also was observed with the addition of just 15% ethanol. Kitawaga suggested that ethanol and l-menthol stimulated the skin penetration of hydrophilic parabens in part due to SC lipid lamella fluidization. The decrease of BP permeation could have been due to the increase of BP solubility in the presence of ethanol in the vehicle, thus reducing BP partitioning between the skin and the vehicle.
The effect of the N-dodecyl-2-pyrrolidone (ND2P) on paraben penetration also was studied and found to stimulate the permeation of hydrophilic parabens, in particular MP with Log Pow of less than 2. ND2P had the same effect as ethanol on the SC lipid lamella; it perturbs the lipids by increasing their fluidization, thus enhancing hydrophilic paraben permeation.17
Paraben Combinations
Caon also compared the penetration of a single paraben to the penetration of combined parabens. The binary combination of PP with three other parabens reduced flux values, particularly when PP was combined with EP. Caon proposed this to be due to physicochemical properties since the PP binary combination with EP decreased solubility by 50% and by only 10% with MP and BP in water. This flux decrease could be explained by the change of solubility that could modify the partition coefficient of parabens within the skin layers.11
Effects of Reapplication, Occlusion on Penetration
El Hussein et al. investigated the effects of reapplying a product containing parabens during the course of a day. The researchers applied a commercial body lotion containing MP, EP, PP and BP at 0 hr, 12 hr and 24 hr and the concentration in the liquid receptor was measured before each reapplication at 12 hr, 24 hr and at 36 hr. The penetration order still followed the lipophilicity parameters with the exception of PP, which had a higher concentration in the lotion than EP. The total parabens liberated in the liquid receptor were higher after reapplication of cosmetics containing parabens, compared with a single application.20 Thus, various paraben concentrations can modify the lipophilic order.
Occlusion is also a factor to consider when assessing paraben penetration, as clothes or accessories covering the skin can modulate penetration. Occlusion was studied ex vivo by Cross with different paraben vehicles after the placement of a piece of high density polyethylene on human epidermis. A significant change in the epidermal flux of parabens was noted following occlusion. Increases were observed for acetone and ethanol vehicles, and a decrease was seen following occlusion for the ointment formulation. For the ointment, changes in flux following occlusion appeared to result from a significant decrease in paraben epidermal partitioning and from an increase in paraben epidermal diffusivity by the solvent vehicles. These studies show that paraben penetration is vehicle-dependent with or without occlusion.12 The choice of an ointment base for body care products that consumers apply beneath clothes, such as a lotion, could therefore be advantageous to avoid paraben penetration.
In vivo Paraben Pharmacokinetic Studies
Janjua and Tanaka studied paraben concentration in human urine before and after the application of a formulation containing parabens. Janjua focused on the systemic absorption of BP in 26 male volunteers by applying to the whole body, every day under the same conditions, 2 mg/cm2 of a cream without parabens for one week (control), followed by applying the same amount of a cream containing 2% BP the next week. Serum and urine samples were collected every 24 hr. Serum BP was undetectable during the control week but its concentration peak (130 μg/L) was detected 3 hr after the first application of the BP cream, showing rapid skin penetration and a systemic uptake.
Urine samples were collected every day and BP urine concentration remained constant during the treatment week (25 μg/L); 24-hr excretion profiles were then calculated and mean flux of 32 μg/cm2/hr was demonstrated. Reproductive and thyroid hormones levels were also analyzed in the blood sample, and no biologically significant effects were observed. The urine concentration of BP was under the limit of detection during the control week but this increased by a thousand times during the treatment week, although only 0.9% of the applied amount was recovered.21, 22
It should be noted that for single parabens, the amount permitted in cosmetics is 0.4% and Janjua applied a cream containing six times this amount. In addition, the cream thickness tested on the skin—i.e., 2mg/cm2—was higher than the amount typically applied by consumers.23 It would thus be relevant to study paraben recovery in vivo with products available on the market and to allow volunteers to apply the product in accordance to their daily practices.
In vivo cyclodextrin still greatly reduced paraben absorption; a patch test containing either radio-labelled MP [14C], MP alone, or MP with radio-labelled cyclodextrin [14C]HP-C-Cyd was applied on the dorsal skin of hairless mice for 24 hr and in the presence of cyclodextrin, MP was significantly retained in the patch. The paraben amounts excreted in urine, feces and lung respiration were significantly reduced by 66% in the presence of [14C]HP-C-Cyd. In this study, Tanaka suggested that only the free fraction of MP in equilibrium with the complexed fraction was percutaneously absorbed.16
Paraben Risk Analysis
Parabens are generally considered safe preservatives because after penetrating the SC, they are converted by carboxylesterases into HBA, a less toxic metabolite. Carboxylesterase is present in the epidermis and dermis, and it hydrolyzes MP and EP into HBA more than other parabens;24, 25 in fact, in a study by Ishiwatari, only 0.028% of MP was found to remain unmetabolized on the skin surface 12 hr after application.26 It can therefore be concluded that MP and EP are less toxic than the more lipophilic parabens after they penetrate the SC.
Consumer fears over parabens began when researchers demonstrated them to exhibit estrogenic activity, which could be linked to various diseases such as breast cancer, or affect the male reproductive system. Darbre in particular found parabens in human breast tissues, although no control tissues were examined in the study, making this link between parabens and breast cancer unfouded.1 Many studies have found parabens to exhibit weakly estrogenic activity, which increases with the alkyl group size—i.e., BP is 10,000 times less potent than 17b-estradiol; MP is 2,500,000 times less potent than 17b-estradiol; EP is 150,000 times less potent than 17b-estradiol; and PP is 30,000 times less potent than 17b-estradiol. HBA was found to be inactive.27 Despite these findings, however, Golden observed that if consumers were to use cosmetics containing BP every day, the total daily BP dose would be approximately 0.12–0.41 mg/kg/day, which corresponds to a BP daily intake of between 1,500 and 5,000 times less than the minimal amount shown in an in vivo assay to stimulate estrogen activity.28
On the other hand, a study by Prusakiewicz et al.2 showed that BP and PP were able to increase estrogen activity by inhibiting estrogen sulfotransferases in skin, thus stimulating or prolonging estrogen signaling cascades. In this study, the paraben concentration used was equivalent to what could penetrate from 1% of a cosmetic formulation containing less than 1% parabens. Considering that less BP and PP are converted into HBA than MP and EP, it would be necessary to study this phenomenon more.
Contact allergy is another consideration, although parabens rarely cause this condition. In fact, 70 years of paraben use have shown that they are non-irritating preservatives.29 However, Fisher highlighted the “paraben paradox” in which paraben-sensitive skin in a normal state was not found to react to the application of a cosmetic containing parabens, but when the skin was traumatized or eczematous, it easily reacted to parabens. Therefore, these preservatives are well-tolerated by skin in a normal state, even if the skin is sensitive to parabens.30
Paraben Alternatives
As noted, since it was communicated that parabens may potentially exhibit estrogenic effects, consumers have feared them and marketers have listened to consumers. In response, the current trend is to remove parabens from cosmetics and to promote self-preserved products claiming to be paraben- or preservative-free. In addition, marketers have replaced parabens with natural preservatives or cosmetic ingredients having antimicrobial properties that are not registered as preservatives in order to satisfy the consumer. These alternative preservation systems are called hurdle technologies, as first defined by Leistner for food applications.31 Such systems account for the product manufacturing process, packaging, emulsion formation, water activity, pH control and multifunctional antimicrobial ingredients.
For example, preservative-free cosmetics must be manufactured in strictly aseptic conditions. In addition, protective packaging such as tubes (preferably metal) are preferred over wide-mouthed jars. These conditions alone demonstrate the microbial challenges posed by multiple finger contacts. The metal tube does not allow for air to be drawn in after dispensing the product; similarly, a pump also limits finger contamination. Preferable packaging would be an airless pump for the same reasons as the metal tube; however, costs must be considered and manufacturers must ensure that consumers will pay more money for the same cosmetic in different packaging. The most secure packaging is the single application pack or single shot capsule since it is a perfect aseptic environment, but it is not ecological because the consumer must throw away the package after each use. Besides packaging, other methods to avoid microbial contamination include lowering the water activity of the product with water-binding substances and favoring o/w emulsions. Also, pH levels < 4 or > 10 reduce microbial growth, although they can cause skin irritation.3, 32
Alternative antimicrobial ingredients that are not registered as preservatives, such as caprylyl glycol and glyceryl caprylate, are only effective in combination with other ingredients. For example, natural ingredients like lonicera extracts seem promising for the development of self-preserved products but require the addition of glyceryl caprylate, levulinic acid, p-anisic acid and ethanol. In addition, organic acids must be combined with diazolidinyl urea.33
Finally, self-preserved formulations require stringent microbiological and stability testing to ensure their effectiveness because the utilization of such alternative or natural substances does not ensure complete elimination of adverse events, irritating effects or sensitization. To replace parabens with natural preservatives is not necessarily preferable since natural ingredients are not suitable for all consumers because they themselves can contain impurities that can cause allergic reactions.4
Conclusion
This review has highlighted two key points regarding paraben percutaneous penetration factors. First is that the more soluble parabens are in a formulation, the more they are retained and the less they penetrate. Second is that lipophilic parabens are retained by the SC lipids and it is difficult for those parabens to cross this barrier, whereas more hydrophilic parabens can cross this barrier into the upper epidermis, although most are bioconverted into HBA. It is also difficult to generalize the diffusion properties of just one paraben or combination of parabens because the whole formulation must be taken into account.
Penetration results from ex vivo studies depend upon operative conditions, skin origin, skin treatment, heat separation, etc., as well as cutaneous barrier status. And while in vivo experiments such as the studies by Janjua and Tanaka could be a solution, they are no longer viable trends due to strict regulations and the ban on animal testing.23, 24 Further, every formulation needs to be tested but it is complicated to do so, and published mathematical models (often polynomial functions) are not sufficient. An interesting alternative would be to establish physicochemical factors that would lead to a complete mathematical penetration model. This model could account for the formulation and the skin origin and not just the Log Pow and Mw.
The presence of preservatives in cosmetic formulations is controversial and the ideal solution to replacing traditional/chemical preservatives with absolutely safe, effective and compatible materials for all applications has not yet been found. In relation, consumers who avoid preservatives should be educated about cosmetic contamination, especially for cosmetics created in the home; the only secure way to avoid microbiological contamination is to use well-known preservatives. Finally, consumers should be made aware that newer natural preservatives may not have been tested to the extent of parabens, thus penetration data likely does not exist yet for them.
References
Send e-mail to [email protected].
1. PD Darbre, A Aljarrah, WR Miller, NG Coldham, MJ Sauer and GS Pope, Concentrations of parabens in human breast tumors, J Appl Toxicol 24(1) 5–13 (2004)
2. JJ Prusakiewicz, HM Harville, Y Zhang, C Ackermann and RL Voorman, Parabens inhibit human skin estrogen sulfotransferase activity: Possible link to paraben estrogenic effects, Toxicology 232(3) 248–256 (2007)
3. A Varvaresou et al, Self-preserving cosmetics, Int J Cosmet Sci 31 163–175 (2009)
4. MS Reish, Preservatives under fire, Chem Eng News 88(20) 13–6 (2010)
5. D Steinberg, Common Preservatives, ch 2 in Preservatives for Cosmetics, 2nd edn, Allured Business Media: Carol Stream, IL, USA (2006) 11–14
6. MG Soni, IG Carabin and GA Burdock, Safety assessment of esters of p-hydroxybenzoic acid (parabens), Food Chem Toxicol 44(7) 985–1015 (2005)
7. MD Lundov, L Moesby, C Zachariae and JD Johansen, Contamination versus preservation of cosmetics: A review on legislation, usage, infections and contact allergy, Contact Dermatitis 60(2) 70–78 (2009)
8. Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products, Int J Toxicol 27 suppl 4, 1–82 (2008)
9. Organization for Economic Co-operation and development, (2010) Guidance document for the conduct of skin absorption studies, www.oecd.org/dataoecd/0/1/46257610.pdf (Accessed Feb 2, 2011)
10. A Dal Pozzo and N Pastori, Percutaneous absorption of parabens from cosmetic formulations, Int J Cosm Sci 18(2) 57–66 (1996)
11. T Caon, AC Costa, MA De Oliveira, GA Micke and CM Simoes, Evaluation of the transdermal permeation of different paraben combinations through a pig ear skin model, Int J Pharm 391 1–6 (2010)
12. SE Cross and MS Roberts, The effect of occlusion on epidermal penetration of parabens from a commercial allergy test ointment, acetone and ethanol vehicles, J Invest Dermatol 115(5) 914–918 (2000)
13. C Merle and A Baillet-Guffroy, Physical and chemical perturbations of the supramolecular organization of the stratum corneum lipids: In vitro to ex vivo study, Biochimica et Biophysica Acta 1788(5) 1092–1098 (2008)
14. SY Oh et al, The effect of ethanol on the simultaneous transport and metabolism of methyl p-hydroxybenzoate in excised skin of Yucatan micropig, Int J Pharm 236(1–2) 35–42 (2002)
15. E Esposito, F Bortolotti, C Nastruzzi, E Menegatti and R Cortesi, Diffusion of preservatives from topical dosage forms: A comparative study, J Cosmet Sci 54(3) 239–250 (2003)
16. M Tanaka et al, Effect of 2-hydroxypropyl-beta-cyclodextrin on percutaneous absorption of methylparaben, J Pharm Pharmacol 47(11) 897–900 (1995)
17. S Kitagawa, H Li and S Sato, Skin permeation of parabens in excised guinea pig dorsal skin, its modification by penetration enhancers and their relationship with n-octanol/water partition coefficients, Chem Pharm Bull (Tokyo) 45(8) 1354–1357 (1993)
18. S Nicoli, F Zani, S Bilzi, R Bettini and P Santi, Association of nicotinamide with parabens: Effect on solubility, partition and transdermal permeation, Eur J Pharm Biopharm 69(2) 613–621 (2008)
19. WJ Romonchuk and AL Bunge, Mechanism of enhanced dermal permeation of 4-cyanophenol and methylparaben from saturated aqueous solutions containing both solutes, Skin Pharmacol Physiol 23(3) 152–163 (2010)
20. S El Hussein, P Muret, M Berard, S Makki, P Humbert, Assessment of principal parabens used in cosmetics after their passage through human epidermis-dermis layers (ex-vivo study), Exp Dermatol 17(8) 700–701 (2008)
21. NR Janjua, GK Mortensen, AM Andersson, B Kongshoj, NE Skakkebaek and HC Wulf, Systemic uptake of diethyl phthalate, dibutyl phthalate, and butyl paraben following whole-body topical application and reproductive and thyroid hormone levels in humans, Environ Sci Technol 41(15) 5564–5570 (2007)
22. NR Janjua, H Frederiksen, NE Skakkebaek, HC Wulf and AM Andersson, Urinary excretion of phthalates and paraben after repeated whole-body topical application in humans, Int J Androl 31(2) 118–130 (2008)
23. E Jungman, HI Maibach, Enhancing sunscreen efficacy for realistic application, Cosm & Toil 125(7) 28–30 (2010)
24. C Jewell et al, Hydrolysis of a series of parabens by skin microsomes and cytosol from human and minipigs and in whole skin in short-term culture, Toxicol Appl Pharmacol 225(2) 221–228 (2007)
25. C Lobemeier, C Tschoetschel, S Westie, E Heymann, Hydrolysis of parabens by extracts from differing layers of human skin, Biol Chem 377(10) 647–651 (1996)
26. S Ishiwatari, T Suzuki, T Hitomi, T Yoshino, S Matsukuma and T Tsuji, Effects of methyl-paraben on skin keratinocytes, J Appl Toxicol 27(1) 1–9 (2007)
27. EJ Routledge, J Parker, J Odum, J Ashby and JP Sumpter, Some alkyl hydroxy benzoate preservatives (parabens) are estrogenic, Toxicol Appl Pharmacol 153(1) 12–19 (1998)
28. R Golden, J Gandy and G Vollmer, A review of the endocrine activity of parabens and implications for potential risks to human health, Crit Rev Toxicol 35(5) 435–458 (2005)
29. D Sasseville, Hypersensitivity to preservatives, Dermatol Ther 17(3) 251–263 (2004)
30. AA Fisher, The parabens: Paradoxical preservatives, Cutis 52(5) 254–256 (1993)
31. L Leistner, Basic aspects of food preservation by hurdle technology, Int J Food Microbiol 55(1–3) 181–186 (2000)
32. AC Dweck, Natural Preservatives, ch 49 in Naturals and Organics in Cosmetics: From R&D to the Market Place, Allured Business Media: Carol Stream, IL, USA (2007) pp 453–462
33. K Weber, New Alternatives to Paraben-Based Preservatives Blends, Cosm & Toil 120(1) 57–61 (2005)