Every year, around October, children begin dreaming of the gifts they want for the holidays and put them on their wish lists. Due to the economy, however, many children were disappointed in 2009. Like the children, the US Food and Drug Administration (FDA), along with other federal agencies, publishes its wish list called the Semiannual Regulatory Agenda. The Regulatory Flexibility Act of 1980 and Executive Order 12866 require the publication of this semiannual inventory of the rulemaking under development, and the US Department of Health and Human Services (HHS) publishes this wish list for the FDA. The latest came out on Dec. 7, 2009. This author always notices when the list comes out since the questions begin regarding the dates on the list, especially those asking whether the dates are for real. Like all wishes and dreams, sometimes they do come true, but the FDA’s batting average is well below the Mendoza Line—i.e., less than 200, or 1 in 5 (which, of course, is poor).
Most of the trouble with the Unified Agenda is caused when someone from the FDA speaks publically and is asked far-reaching questions such as, “When will the Final Monograph on insect drugs be issued?” to which the speaker responds with what items are included in the Unified Agenda. Once audience members hear the agenda, they expect those items to be acted upon and when they are not, like the children, they are disappointed. Therefore, please note that the following items are listed in the Unified Agenda but this should not be used as a basis for marketing or sales plans; while on the agenda, they are rarely completed, as will be shown.
The Latest Unified Agenda
Of all the agenda items, following are the four that are relevant to personal care, in the order in which they appear. With regard to the FDA’s language throughout, it should be noted that a final action refers to issuing a Final Rule on a given topic; anything else is merely proposed.
1. Prerule stage: Item one on the Unified Agenda, in the “prerule stage,” addresses OTC drug labeling requirements. In 1999, the FDA published its final rule stating that a standardized labeling format would significantly improve the readability of OTC labels by familiarizing consumers with the information included and the location of the information. In addition, a standardized appearance with standardized content, including user-friendly visual cues, would help consumers to locate important health and safety information, thus allowing them to quickly and effectively compare and select products. This became known as the “Drug Facts” box, which is required for all OTC drugs with the exception of the current stay for new sunscreens.
Following this decision, the FDA is initiating a review of these rules to determine whether they should continue without change or whether they should be further amended or rescinded to minimize adverse impacts on a substantial number of small entities. The FDA is soliciting information and will consider comments on the following:
- The continued need for the regulation in part 21 CFR 201.66;
- The nature of the complaints or comments from the industry received by the FDA concerning the regulations;
- The complexity of the regulations;
- The extent to which the regulation overlaps, duplicates or conflicts with other federal, state or governmental rules; and
- The degree to which technology, economic conditions or other factors have changed for the products, which are still subject to the labeling regulations.
2. Proposed rule stage: Items listed as being in the “proposed rule stage” on the agenda, including sunscreen products, were expected to be completed by the end of February 2010 based on the last Unified Agenda issued in May 2009. The first action on the agenda addressed products containing the combination of sunscreen and insect repellent ingredients. The second action addressed active ingredients under Time and Extent Application (TEA) review while the third action addressed other efficacy issues for OTC sunscreen drug products. Finally, the fourth action addressed sunscreen formulation, labeling and testing requirements for both UVB and UVA radiation protection.
The timetable for the completion of proposed rules for combined sunscreens and insect repellents is yet to be determined (TBD). Regarding the development of proposed rules for sunscreen efficacy issues on UVA/UVB protection, May 2010 is the expected deadline. In the case of active ingredients under TEA review, no date was given, which could mean the TEAs may be issued at any time.
3. Final rule stage: Five agenda items relevant to personal care fell within the “final rule” stage.
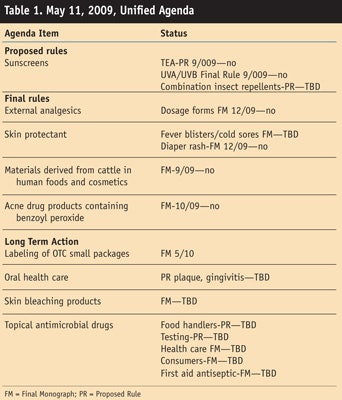
1) External analgesic products: The Final Monograph addresses the 2003 proposed rule on patches, plasters and poultices and the related proposed rule will address issues not addressed in previous rulemakings. The timetable for the final action on external analgesic products—i.e., by which dosage forms are expected to be completed—is September 2010. For proposed rules for other issues regarding these products, the timetable is TBD.
2) Labeling of small-sized OTC drugs: The final action item addressing labeling for convenience or small-sized OTC drug packages is expected to be completed by May 2010.
3) Skin protectant products: The first action for skin protectant products addresses those used to treat fever blisters and cold sores, with an expected timetable for completion of May 2010. The second action, identifying safe and effective skin protectant active ingredients to treat and prevent diaper rash, is expected to be completed by June 2010.
4) Acne drug products containing benzoyl peroxide: This action item to address acne drug products containing benzoyl peroxide was expected to be completed by December 2009—which as of press, has long passed without action.
5) Materials derived from cattle in human foods and cosmetics: On July 14, 2004, the FDA issued an interim final rule (IFR) that was effective immediately to prohibit the use of certain cattle material and to address the potential risk of Bovine spongiform encephalopathy (BSE) in human food, including dietary supplements and cosmetics.
On April 17, 2008, the FDA amended the IFR so that it could designate a country as not being subject to certain BSE-related restrictions relating to prohibited cattle materials applicable to human food and cosmetics. Comments submitted in response to the July 14, 2004, IFR that were not addressed in the Sept. 7, 2005, and April 17, 2008, amendments will be addressed in the final rule. The final rule, expected to be completed by October 2010, will also respond to comments submitted following the Sept. 7, 2005, and April 17, 2008, amendments.
4. Long term actions: Long term actions noted on the agenda include oral care, skin bleaching and topical antimicrobials. With regard to oral health care products, the notice of proposed rulemaking and final action will address oral health care products used to reduce or prevent dental plaque and gingivitis; the timetable for this item is TBD. For skin bleaching products, this action will address drug products containing hydroquinone, also at a time TBD. For topical antimicrobial drug products, the first action addresses health care products.
The second action relates to food handler products and the third action addresses testing requirements. Finally, the fourth action addresses consumer products. The final actions listed will address health care, consumer and first aid antiseptic drug products, respectively. Except for the fourth action relating to consumer products, these issues are expected to be completed by December 2010.
Comments
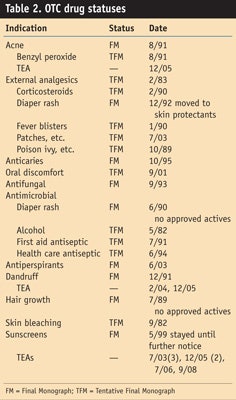
To prove that these items are mere wishes on the FDA’s latest list, some evidence is required, which the previous Unified Agenda, issued on May 11, 2009, provides. As can be seen in Table 1, none of the action items from the previous Unified Agenda were completed; although those marked TBD will happen at some undisclosed time. In relation it may also be helpful for the reader to see where all the OTC drugs are at in the process, which was only supposed to take 6 years to complete but was started in the early 1970s (see Table 2).
It is clear that the FDA has significant work to do to in order complete these issues. The failure to advance TEAs makes little sense, especially in the case of sunscreens; the TEA process begins by the FDA comparing a submission with a simple check-off list to determine whether the requirements for the submission are met. This process should take no more than 5 min to complete since the submission either has the correct information or does not. However, the FDA typically takes a year to complete this process.
After the submission has been found to meet the requirements and is approved, a call goes out for safety and efficacy data. These are the dates listed in Tables 1 and 2. To date, none of these TEAs have advanced past this stage.When it comes to sunscreens, the process should be simple since no efficacy data is needed for the submission. As the FDA states in its Final Monograph for sunscreens, efficacy, as defined by SPF testing, is determined on the final formulation. Further, each individual active in the sunscreen must increase the SPF by 2. Therefore, efficacy is not an issue because if the active does not work in a formulation, the product manufacturer cannot use it. This is why many Category I filters are either not used or are not even produced—they simply do not work.
Removing efficacy as an issue leaves only the safety of UV filters for consideration. In this author’s opinion, if the FDA lacks the time and personnel to review the safety data, a simple solution would be to outsource this function and review the reports. This lack of approvals for second and third generation UV filters has placed the United States as the least progressive market in offering consumers effective UVA and UVB protection. Recall, however, at the beginning of this column, that the Unified Agenda was not described as a list of when the FDA will address these concerns and issue new rules; it really is more of a wish list and should not be taken as more than that.
Alcohol
While researching the information for this column, this author came across an important and interesting proposed rule that was issued in May 1982 with no further action. This rule relates to the use of alcohol in topical OTC drugs. Drug actives are required to be monographed in the US Pharmacoepia (USP) and must meet these standards. Alcohol, per the USP, is listed as being 94.9% to 96% pure alcohol by volume. There is a monograph called “Rubbing Alcohol,” which is denatured alcohol—either SD alcohol 23-H or 40-A or B at different percentages of actual alcohol.
The major use of alcohol is in “instant skin sanitizers,” which is based on the TFM of 1994 requiring 60% to 95% alcohol that is denatured to meet any Alcohol, Tobacco and Firearms-listed products.
While it is clear from the intent of both Federal Register publications that for topical use, one must use denatured alcohol, a manufacturer would either be mislabeling a product by using the word alcohol as the active ingredient, which would not be USP, or would be in violation of the TFM if using the correct USP designation of rubbing alcohol, since it is not approved.
The simple solution would be to use the correct drug word and specification of alcohol and then list the denaturants in the inactive ingredient listing. However, these are mere suggestions and legal advice should be sought on this matter. Wouldn’t it be wonderful if the FDA cleared up this confusion?