In life, hair is one of the most immediately perceived attributes of beauty. For this reason, individuals want their hair to look healthy, shiny and soft. As the use of at-home treatments such as bleaches, dyes and permanent waves grows, extensive hair damage often results. Thus, caring for and repairing damaged hair is an important need, and designing shampoos that specifically treat damaged hair is a constant challenge for formulators.
Micellar shampoos consisting of surfactant blends that form simple micelles constitute the majority of mass market formulations. This is because they are relatively inexpensive to formulate since their principal ingredients, including sodium laureth sulfate and cocamidopropyl betaine, are major commodity ingredients. On virgin hair, their performances are satisfactory.
With the help of conditioning polymers and silicone oils like dimethicone, they cleanse and condition efficiently and provide ease of combing as well as manageability. But while continued efforts are made to improve them, micellar shampoos offer limited performance on damaged hair. Specifically, their ability to deposit silicone oil and provide ease of combing and conditioning efficacy can be significantly reduced, as will be illustrated in the present article.
Structured formulations including specifically chosen combinations of surfactants1 will form micron-sized, close-packed objects referred to as multilamellar vesicles (see Figure 1). These vesicles are stacked in concentric, charged bilayers2 separated by layers of water and supported by electrostatic repulsions. These objects, swollen with water, fill out the formulation space, co-existing and interacting with other ingredients such as oils and polymeric conditioners. Under a well-controlled and simple production process, their production can be scaled up from the laboratory to an industrial plant.
The present article explores how formulators and consumers can benefit from structured rather than micellar shampoos. During application, structured shampoos exhibit a characteristic creamy texture that appeals to consumers seeking rich sensorial experiences. Formulators can use such systems to design chassis that better stabilize and deliver different types of actives to equally repair, treat or protect virgin, damaged, ethnic or colored hair, thus paving the way to a new generation of high-performance shampoos.3
Materials and Methods
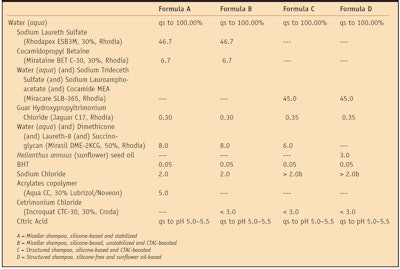
The performances of shampoos incorporating 45 parts by weight (unless stated otherwise) of a specifically chosen surfactant combinationa were systematically compared to micellar shampoos incorporating sodium laureth sulfate and cocamidopropyl betaine, respectively 14 and 2 parts by weight of active surfactants. Both shampoo chassis also incorporated 0.3% to 0.35% conditioning cationic guarb, and 3.0% to 4.0% conditioning oils such as silicone (dimethicone) oilc or Helianthus annuus (sunflower) seed oild.
To stabilize the micellar chassis against creaming or phase separation of the oil, 1.5% w/w acrylates copolymere was added as rheological control agent in some cases. Sodium chloride also was added as needed for each type of structure; up to 3.5% w/w for structured shampoos and 2.0% w/w for micellar shampoos, to adjust the viscosity of the formulas to the typical 10,000 mPas at 10 rpm, spindle 5, as measured with a viscosimeterf. Optionally, a cationic ingredient like cetrimonium chlorideg (CTAC) could be added to formulations to boost performance, as will be detailed further below.
Table 1 summarizes the various formulations used for the described studies. The four main types include: A) a silicone-based micellar shampoo stabilized with a rheological agent; B) a non-stabilized version of the same boosted with increasing amounts of a cationic surfactant; C) silicone-based structured shampoos boosted with increasing amounts of the same cationic ingredient; and D) the silicone-free version of a structured shampoo. Readers should note that the structured formulations used for the present studies have all passed the classical 45°C, three-month stability test (data available upon request) and that no macroscopic phase separation was observed between surfactants, water and oil.
In-house evaluations including dimethicone deposition,4 combing benefitsh and color fadej were carried out by applying a 0.9-g sample of shampoo to 4.5-g hair tressesk (unless stated otherwise), either virgin or damaged by a single- or double-bleaching procedure. Specific evaluations such as salon half-head tests by professional hairdressers were carried out independentlym.
Silicone Oil Deposition
When actives to condition or treat hair require suspending, close-packed multilamellar vesicles bring yield to a formulation without the need for rheological agents. On the contrary, such rheological materials are generally necessary in micellar shampoos. As an illustration, a micellar shampoo containing anti-dandruff particles, color pigments or emulsion droplets generally does not pass the classic 45°C, three-month stability test; macroscopic phase separation of the active is observed over time. Formulators could attempt to match the density of the medium to that of the active to suspend it but while this would likely slow the phase separation down, it would not prevent it; especially if the sample were exposed to even slightly higher temperatures where this density-matching would not hold.
In two-in-one micellar shampoos, acrylates copolymers are often used to suspend and stabilize oil droplets but these anionic copolymers have a measurable impact on ability of micellar shampoos to deposit dimethicone emulsions on hair. This impact is obvious when one compares the deposition ability of the stabilized micellar formula A from Table 1 (see Figure 2), with the unstabilized micellar formula B from Table 1 (see Figure 3a). A plausible reason for this could be that anionic copolymers participate in and alter the well-known flocculation mechanism between surfactants and conditioning cationic polymers, thus reducing the amount of oil deposited.
However, bringing yield to a formulation is the most common way to stabilize a dispersion and make it more durable. The structured shampoo formula C from Table 1, in its non-CTAC boosted version, imparts yield sufficient to stabilize oil and provides better deposition of dimethicone on virgin hair, in comparison with the micellar shampoo formula A from Table 1, which is stabilized with acrylates copolymer (see Figure 2). This enhanced deposition of silicone oil can also be evidenced on damaged hair (also see Figure 2), yet at too small a level to ensure effective conditioning. While virgin hair is hydrophobic and neutral overall, chemically modifying hair by bleaching, perming or dyeing results in damage and hydrophilic and significantly anionic regions.5 Beyond creating a formulation with superior care for virgin hair, designing shampoos that are equally able to condition such different types of hair is clearly a challenge.
Due to their interaction with anionic surfaces like damaged hair, cationic surfactants such as cetrimonium chloride are often used; and in spite of their cationic nature, they can be formulated into mainly anionic shampoos with no real difficulty and at reasonable levels—i.e., on the order of less than 1.0% to 2.0% w/w. However, these ingredients again have a measurably negative impact on the level of conditioning provided by a micellar shampoo (see Figure 3a): The amount of dimethicone deposited by the non-stabilized micellar shampoo formula B from Table 1 on virgin and bleached hair decreases when the amount of CTAC increases, in spite of the presence of a conditioning guar.
On the contrary, the structured shampoo formula C from Table 1 shows a constant and significant increase in the amount of dimethicone deposited on both virgin and damaged hair (see Figure 3b)—two to seven times more on damaged hair than classic micellar shampoos under similar conditions. The treatment of damaged hair is clearly favored, as is shown by Figure 4 where the ratio of damaged/virgin deposition increases with a CTAC-boosted structured shampoo while it stagnates with a micellar one.
Structuring two-in-one shampoos as multilamellar vesicles therefore boosts deposition where it is needed most. Trials with other cationic agents at the same concentration and with the same process of introduction demonstrated the universality of the synergy between structured systems and cationic surfactants, offering alternatives to the formulator such as the more efficient quaternized agent stearalkonium chloriden (SAkC) or the quat-free stearamidopropyl dimethylaminp (SAPDIMA).
Stabilizing and Delivering Natural Oils
When it comes to developing silicone-free shampoos for specific claims, structured systems can be used to stabilize and deliver natural oils without the need to pre-emulsify them. The yield is sufficient to successfully emulsify and stabilize oils as different as sunflower, apricot or petrolatum, producing formulations that pass the standard 45°C, three-month stability test. The successful deposition of sunflower oil on damaged hair, for instance, can be indirectly evidenced by combing experiments.
Highly damaged tresses, i.e. twice-bleached, were first combed in their original dry or wet state, prior to shampooing. Once washed with a structured shampoo containing the 3.0% w/w sunflower oil formula D from Table 1, or 3.0% w/w dimethicone for comparison, the tresses were combed again to deduce the percentage of reduction in the work necessary to comb them in their wet and dry states. These measurements demonstrate that the shampoo based on sunflower oil can make hair easier to comb to the same or greater extent as dimethicone on wet hair just after shampooing (see Figure 5a).
Once hair has been dried, the sunflower oil-based structured shampoo also showed superior performance on damaged hair, a result later confirmed by a half-head salon testing carried out by hairdressers on 10 female subjects with damaged hair. The feel and combability (see Figure 5b) of dry, damaged hair conditioned with 3.0% w/w emulsified sunflower oil, deposited via a structured shampoo, exceeded a leading 3.0% w/w dimethicone-based brand even in its non-CTAC-boosted version.
A natural oil structured shampoo can thus provide conditioning performance that is equal to or better than that of a silicone-based shampoo. In addition, the structured shampoo imparted no measurable buildup on hair, as was verified through 5-day build-up studies carried out independentlym. Similar stabilization and delivery benefits were found to apply to other soothing and treating oils such as meadowfoam, and apricot oils or petrolatum, the latter being particularly suitable for nourishing and moisturizing dry, ethnic hair.
Color Protection
Color protection shampoos have been around for more than 20 years and numerous references to them are found in the literature. In practice, the main cause of color fade is the water used during hair washing.6, 7 Each shade used to produce the final color can be affected by color fade; from individual changes in the three L, a, b color coordinates to overall fade ΔE = √ (ΔL² + Δa² + Δb²) (see Figure 6a). These changes are easily perceived by consumers. An increase in coordinate b coincides with the perceived yellowing of hair, whereas an increase in L gives the impression of duller-looking color. Any hair dye, even a permanent one, is vulnerable to such washout and unfortunately, the more damaged hair is prior to coloring, the faster the color fades away. Even a single shampooing of double-bleached, Caucasian hair dyed with an auburn kit can result in a ΔE on the order of 10 units. And since the extent of hair damage is, again, key to protecting artificial color, shampoos designed specifically for the care of damaged hair may improve color retention.
Much attention has been paid to the potential of conditioning polymers and silicones to protect color but little work has been published on the effects of surfactants to protect color; and none on the benefits of structuring cleansing agents as multilamellar vesicles. Thus, here the authors compared the extent of color fade caused by one surfactant system before and after being structured as multilamellar vesicles (see Figure 6b). The surfactant micellar state, i.e. at approximately 22% w/w raw surfactant in pure water, yielded a global color loss on the order of a ΔE = 8.7 after three shampoo cycles, performed on extensively damaged, twice-bleached hair tresses dyed with a leading international brand auburn color kit. Such fading would likely give consumers the impression of fast color fading.
However, after the surfactant mixture was structured as multilamellar vesicles, the color fading was reduced by nearly 48%; i.e., ΔE = 4.8. This effect is noticeable to consumers since the human eye can perceive color differences as small as a ΔE ~2 for dark tones, and even < 1 for lighter tones. Remarkably, in a complete formulation—i.e., multilamellar vesicles mixed with conditioning polymers, oils, perfumes and preservatives—this reduction in color fade was retained at a ΔE = 5.9.
Although formulators take special care in designing mild coloring kits, hair is necessarily damaged, at least to some extent, by the bleaching agents used. Structured shampoos with multilamellar vesicles encapsulating surfactants thus seem suitable for designing formulations that reduce color fading.
Enhanced Deposition of Colored Pigments
Besides color retention, structured systems can also enable formulators to deliver charged species that are insoluble in water such as colored pigments to revive hair color, or zinc pyrithione for anti-dandruff benefits. The compatibility of structured surfactant systems with such oppositely charged or amphoteric species and the characteristic yield of the multilamellar vesicles they form in water are indeed sufficient to produce a formula with long-term stability and improved deposition efficacy. This ability to deposit actives could be used, for example, to deliver pigments to compensate for the loss of red—a fragile color shade.
To put this ability of structured systems to the test, color fade measurements were carried out on medium brown, double-bleached hair tresses dyed with a reddish kit and shampooed up to 10 times with either tap water, a micellar shampoo, or a structured shampoo containing stabilized red pigmentsq. The structured shampoo delivered the pigments successfully so that the red shade continuously increased with the number of shampoos (see Figure 7b). This effect was rapid and perceivable by the human eye as soon as five shampoos (see Figure 7a), which would give consumers the impression that the color is strengthened and made more vivid.
Conclusions
Surfactant systems that are designed to form multilamellar vesicles can be used to develop next-generation high performance shampoos. These systems outperform existing micellar shampoos since they easily combine with and stabilize actives ranging from molecular to particulate, liquid to solid, and hydrophobic or cationic, to deliver them equally across damaged and virgin zones to deposit the desired ingredients where they are needed. This concept expands an existing range of surfactant systems inherited from body wash design to enable formulators to create innovative formula alternatives, such as sulfate-free or eco-friendly blends with new and different sensorial profiles.
Acknowledgments: A.-G. Dréno, D. Méchineau, D. Lemos, M. De-Wilde, A. Sahouane, R. Desnos, F. Maloum and M. Ait-Azzouzene contributed to the experimental work presented in this article. In addition, the authors with to thank S. Warburton, T. Futterer, L. Hough, E. Gates, H. Lannibois, E. Leroy and S. Catarino for fruitful discussions throughout the project. Finally, the authors are also thankful to Sensient Cosmetic Technologies for providing the pigments used for the section on deposition of colored pigments.
References
Send e-mail to [email protected].
- EU Pat 586275, Composition cosmétique contenant en suspension des particules non hydrosolubles, P-F Derian and C Willemin, assigned to Rhodia (Oct 8, 1997)
- US Pat 5556628, Free-flowing pseudoplastic cosmetic compositions/suspensions, P-F Derian and C Willemin, assigned to Rhodia (Sep 17, 1996)
- Worldwide Pat WO03/055456, Stable surfactant compositions for suspending components, S Frantz, PL Cotrell and SA Warburton, assigned to Rhodia (Jul 10, 2003)
- D Bendejacq, E Leroy and M Airiau, Small-angle x-ray scattering on multilamellar vesicles as rheological agents in personal care formulations, in the proceedings of the 25th IFSCC Congress, Barcelona, Spain (2008)
- US Pat 2006/0135627, Structured surfactant composition, S Frantz, B Sridhar, SA Warburton and PL Cotrell, assigned to Rhodia (Jun 22, 2006)
- C Robbins, Chemical and Physical Behavior of Human Hair, 4th edn, Springer, Verlag, NY (2002), pp 156–157, 398
- C Bouillon and J Wilkinson, The Science of Hair Care, 2nd edn, CRC press, Boca Raton, FL (2005)