Skin constantly is exposed to environmental oxidative stressors such as ultraviolet radiation (UVR), air pollutants, chemical oxidants and aerobic microorganisms.1, 2 Reactive oxygen species (ROS) are considered major contributors to skin aging, cancer and certain skin disorders3 since they react negatively with DNA, proteins and unsaturated fatty acids.4 In addition, while healthy skin possesses an innate antioxidant defense system, ROS and other free radicals or excessive free radical attack can overwhelm the cutaneous antioxidant capacity, depleting the skin’s antioxidants and damaging biomolecules such as lipids, proteins and nucleic acids. This further leads to oxidative damage, skin cancer, immunosuppression and premature skin aging. Therefore, supplying exogenous antioxidants topically to the endogenous antioxidant system is one approach to preventing or minimizing ROS-induced photoaging.5
With growing consumer appeal for natural and organic products, botanical extracts have become among the most commonly used ingredients in OTC antiaging cosmetic preparations. More recently, claims have focused on their antioxidant properties and ability to modulate certain types of environmental damage. Essential oils in particular, traditionally used for aromatic properties, have good penetration into the skin, which enhances their efficacy.6
In the present article, the authors evaluate the chemical composition and biological activities—i.e., antioxidative, antibacterial and antifungal—of wild Amazonian basil Ocimum micranthum Willd. (also known as O. campechianum Mill.) Labiatae essential oil, and compare these properties with those of commercially available common basil Ocimum basilicum and Thymus vulgaris essential oils.7 In addition, in view of their high antioxidant capacities, the oils were further assessed to determine if their functional capacity could be expressed in finished cosmetic products, and whether the type of formulation could influence the expression of their antioxidant activity.
O. micranthum
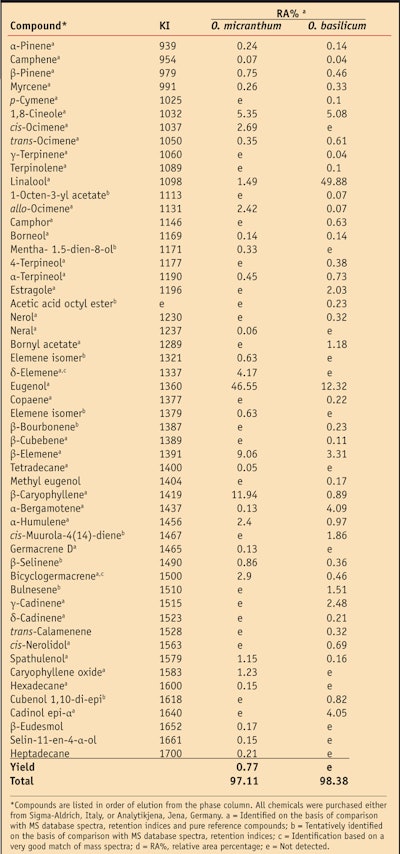
O. micranthum is a lesser known variety of basil native to the South and Central American tropics.8 Also known as Albahaca de campo or Albahaca silvestre, it is widely used by the indigenous population both for culinary and medicinal purposes.9, 10 Specifically, O. micranthum is traditionally used to treat cough, bronchitis and general infections;11 as an anti-inflammatory and to treat conjunctivitis;12 and even as a diuretic and emmenagogue.13 As is common with spices, much of the aroma and flavor of O. micranthum is due to the presence of essential oils. In particular, the main components of O. micranthum essential oil were previously identified as eugenol (46.55 ± 5.11%), β-caryophyllene (11.94 ± 1.31%) and β-elemene (9.06 ± 0.99%), while a small amount of linalool (1.49 ± 0.16%) also was detected.7
Also, as was previously reported,7 the radical scavenging activity of O. micranthum was evaluated employing a 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay that found the oil to exert a good capacity to act as a non-specific donor of hydrogen atoms or electrons, quenching 76.61 ± 0.33% of the test radicals with values higher than those reported by reference oils. In a β-carotene bleaching test, the oil provided antioxidant efficacy comparable with that of O. basilicum and T. vulgaris essential oils. This data was confirmed by photochemiluminescence (PCL), where the oil showed a remarkable antioxidant capacity (2.39 ± 0.1), comparable with that of 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acida and vitamin E, and higher than other essential oils.
Finally, the antibacterial activity of O. micranthum essential oil was evaluated7 against Gram positive and Gram negative bacterial strains, and a dose-dependent antifungal activity against pathogenic and food spoiling yeasts was observed. Thus, as noted, in view of these various functionalities, the application of these essential oils was further examined, as is described here.
Materials and Methods
Plant materials: O. micranthum Willd. (Labiatae) leaves were collected from three different stocks on the outskirts of the Shuar Wasak’entsa reserve in eastern Ecuador (77°.15”W/ 2°.35”S), and a dried specimen of the spice was obtained. In addition, O. basilicum essential oil of linalool chemotype and Egyptian origin was obtained commerciallyb, as was T. vulgaris essential oil of thymol chemotypec. All essential oil samples were stored in glass vials with sealed caps at ± 0.5 °C in the absence of light.
Extraction and isolation: The O. micranthum essential oil was extracted by steam distillation14 for 8 hr to yield 1.54 mL of essential oil from 200 g of crude drug (yield 0.77 or 0.25%); this essential oil content was determined on a volume to dry weight basis. The values for essential oil yield of the three replications were averaged and standard deviations calculated.
Gas chromatography (GC): Essential oil samples from three separate distillations were analyzed and the relative peak areas for individual constituents averaged (see Table 1). The relative percentages were determined using a gas chromatographd equipped with a processore, a flame ionization detector (FID), and a poly-5% diphenyl-95%-dimethyl-siloxane bonded phase columnf (i.d., 0.32 mm; length, 30 m; film thickness, 0.15 μm).
Operating conditions were as follows: injector temperature, 280°C; FID temperature, 280°C; carrier (helium) flow rate, 2 mL/min; and split ratio, 1:40. The oven temperature initially was 45°C, which was raised to 100°C at a rate of 1°C/min; then raised to 250°C at a rate of 5°C/min; and finally held at that temperature for 10 min. A 1-μL aliquot of each sample dissolved in CH2Cl2 was injected. The percentage composition of the oils was computed by the normalization method from the GC peak areas, calculated by mean values of injections from the three batches of O. micranthum essential oil, without using correction factors.
GC/mass spectrometry: The essential oil constituents were analyzed by GC equipped with a mass spectrometer (MS); the constituents of the volatile oils were identified by comparing their GC retention times, Kovats indices (KI) and MS fragmentation patterns with those of known essential oil compositions and pure compounds, as well as MS fragmentation patterns and retention indices with mass spectra libraries and those in the literature.15 The GC conditions were the same as those reported for GC analysis and the same column was used.
The MS conditions were as follows: ionization voltage, 70 eV; emission current, 40 μA; scan rate, 1 scan/sec; mass range, 35–300 Da; and ion source temperature, 200°C. A mixture of aliphatic hydrocarbons (C8-C24) in hexane was injected under the same temperature program described above to calculate the retention indices using the equation generalized by Van del Dool and Kartz.16
Chemicals: The solvents and compounds used as referencesb, c were purchased from both from “General” and “Flavors and Fragrances” catalogues. However, the compounds labeled as tentatively indentified in Table 1 provided retention indices and mass spectra in good agreement with the literature.15
Antioxidant Assessment of Extracts by PCL
The radical scavenging activity of O. basilicum, T. vulgaris and O. micranthum essential oils was evaluated employing PCL assay.17 Briefly, this method involves generating defined free radicals (superoxide anion radicals) in the detection system by exposing a photosensitizer to a UV light source. Free radicals are detected by their reaction with a chemiluminogenic substance and the measurement of the emitted light; the light flashes are detected by a photomultiplier. These generated radicals are partially scavenged by reaction with the sample antioxidants and the remaining radicals are quantified by the above described detection principle. Results are presented in equivalent concentration units of synthetic vitamin Ea for lipid-soluble substances or ascorbic acid for water-soluble substances.
Different concentrations of these standard compounds are used to establish a calibration curve and the detector signal of each run is monitored for 180 sec. The PCL assay is suitable to measure the radical scavenging properties of single antioxidants as well as more complex systems in the nanomolar range.18, 19 The antioxidant potential is measured by means of the lag phase at different concentrations, calculated by a synthetic vitamin Ea calibration curve and expressed as mmol equivalents in antioxidant activity of a reference compound; i.e., synthetic vitamin Ea.
The PCL method was carried out as described by Popov and Lewin;20 in the water-soluble fraction, antioxidants such as flavonoids, ascorbic acid, amino acids, etc. are detected while in the lipid-soluble fraction, tocopherols, tocotrienols, carotenoids, etc. are measured. PCL is simple, quick, sensitive and reliable. Moreover, it is able to evaluate the capacity of tested products to scavenge the radical anion superoxide (O2•–), which is one of the most damaging ROS to human skin.
Antioxidant Assessment of Formulas by PCL
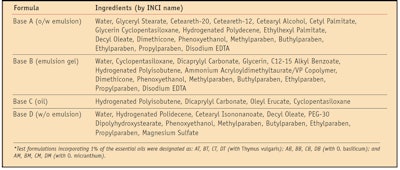
After evaluating the antioxidant capacity of pure essentials oils, a systematic study using PCL was conducted of various essential oil-containing formulations (see Table 2) to determine the efficacy of the oils in topical antiaging applications.
Previous studies by the authors21 have demonstrated that the type of formulation—i.e., w/o, o/w, w/o/w, etc.; the final pH or storage conditions; and the cosmetic base can influence the expression of a functional ingredient’s antioxidant capacity. Therefore, in order to minimize interference with the assessment of the oils of interest, each formulation tested incorporated only emollients, emulsifiers and other excipients without claimed antioxidant activity. Since O. micranthum was uniquely found to be rich in eugenol (46.55 ± 5.11%) (see Table 1), the authors also evaluated the antioxidant capacity of this formula component to compare it with the activity found in the pure oil.
Sample Preparations
A precisely weighted amount of each product in Table 2 was suspended either in: 1 mL of high performance liquid chromatography (HPLC) grade methanol to take measurements using an antioxidant capacity lipid-soluble (ACL) kit; or 1 mL of HPLC grade water for measurement using an antioxidant capacity water-soluble (ACW) kit. Within these setups, each sample was mixed by vortex for 1 min at room temperature and the obtained solution was filtered through an HPLC filterg by a syringe and diluted with Reagent 1 of the ACL or ACW kith.
Different dilutions of the various samples were evaluated to obtain a calibration curve. Results are expressed as mmol equivalents in antioxidant activity of 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acida for each gram of product under examination. The described work was carried out on the cosmetic bases, referred to as A, B, C and D in Table 2, and on the same bases additionally containing 1% of the essential oils. These variations are referred to as: AT, BT, CT, DT (with T. vulgaris); AB, BB, CB and DB (with O. basilicum); and AM, BM, CM and DM (with O. micranthum) in Table 2.
Results and Discussion
Extracts: The evaluation of the antioxidant capacity of pure essential oils indicated that all the oils tested showed the ability, to different extents, to scavenge the radical anion superoxide (O2•–) (see Figure 1). O. micranthum was found to be the most active essential oil—i.e., 15 and 5 times higher than O. basilicum and T. vulgaris, respectively—with an antioxidant capacity value equivalent to 1.65 mmol of 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acida per gram. Although they are grouped in the same genus, O. basilicum and O. micranthum showed different antioxidant capacities. This could be attributed to different chemotypes.
The chemotype of an essential oil is a precise reference that indicates the main distinctive biochemical component present in the oil. It is used to distinguish essential oils extracted from the same botanical variety but with a different biochemical composition. As noted, GC-MS analysis showed O. micranthum to be rich in eugenol (46.55 ± 5.11%) and due to the high antioxidant capacity of eugenol (ACL value = 2.70 mmol 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acida/g), the authors concluded that the different performances of the essential oils could be linked to the variations in content of this component.
Formulations: After preparing various test formulas with and without the essential oils, their antioxidant capacities were determined by PCL. As expected, all the formulations including the essential oils showed an ACL value higher than their respective base alone (see Figure 2, Figure 3 and Figure 4); also, the ACL values of different oil-containing formulations revealed which formulas contained the pure O. micranthum extract (see Figure 1). In fact, all formulations containing O. micrantum as a functional ingredient showed higher antioxidant capacity than the other samples, particularly T. vulgaris.
Thus, the authors concluded that the activity of the essential oils added as functional antioxidant ingredients in finished cosmetic products was maintained in the finished product, although to different degrees. Indeed, the nature, i.e., o/w, w/o, etc., of the formulation was found to significantly influence the ratio of antioxidant capacity expressed even with the addition of the same functional ingredient. In fact, as can be observed from Figure 5, the oil and w/o emulsions show the highest ACL values. For example, the ACL value of one O. micranthum-containing formulation was 16.4 nmol 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acida/mg of cream, with respect to product A, an o/w emulsion, with the ACL value of 9.7 nmol.
This data confirms previous research conducted by the authors using ascorbic acid-containing formulations, which demonstrated that the antioxidant/antiaging properties of finished cosmetic products depended on all the ingredients in the final formulation and not only the functional ingredients. The present work, carried out on different types of formulations, including w/o, o/w, gel emulsions, etc., underlines the fact that the type of formulation used is important for the expression of the antioxidant properties of the functional ingredient added. For additional formula suggestions, see Formula 1, Formula 2 and Formula 3.
Conclusions
The aim of the present work was to examine the antioxidant properties of essential oils, especially Ocimum micranthum Willd. (Labiatae), to determine their functionality in actual applications. In addition to antioxidative and antiradical properties, the essential oil was found to impart antimicrobial activity by inhibiting the growth of food-related yeasts and bacterial contamination.7 In view of the high value of antioxidant capacity of O. micranthum and other essential oils tested, a systematic study of essential oil-containing formulations was then conducted to determine the ability of the oils to express significant activity when added into cosmetic products.
All of the tested products, especially O. micranthum-containing formulations, were shown to be effective but the activity depended upon the type of formulation. Of those examined, w/o emulsions and oils showed higher ACL values. The results proved that some essential oils could be considered valuable cosmeceutical ingredients. However, since the presented data was generated from an in vitro experiment, the antioxidant effects should also be evaluated in vivo in humans. Further studies are under way to verify the radical scavenging activity of other functional antiaging ingredients such as coenzyme Q10, vitamin E and flavonoids, to evaluate their behavior in finished cosmetic products.
References
Send e-mail to [email protected].
- JJ Thiele, F Dreher and L Packer, Antioxidant defense systems in skin, in: P Elsner, HI Maibach, eds., Cosmeceuticals, Drugs versus Cosmetics, New York: Marcel Dekker 145–187 (2000)
- JJ Thiele, Oxidative targets in the stratum corneum. A new basis for antioxidative strategies, Skin Pharmacol Appl Skin Physiol 14 87–91 (2001)
- MA Birch-Machin, The role of mitochondria in aging and carcinogenesis, Clinical and Experimental Dermatology V31, 548–552 (2006)
- F Dreher and HI Maibach, Protective effects of topical antioxidants in humans, in: JJ Thiele and P Elsner, eds., Oxidants and Antioxidants in Cutaneous Biology, Basel: Karger 15–164 (2001)
- M Portugala, V Barakb, I Ginsburgc and R. Kohen, Interplay among oxidants, antioxidants and cytokines in skin disorders: Present status and future considerations, Biomedicine and Pharmacotherapy 61, 412–422 (2007)
- C Krzysztof, Skin penetration of terpenes from essential oils and topical vehicles, Planta Medica 72(4) 311-316 (2006)
- G Sacchetti et al, Composition and functional properties of the essential oil of Amazonian basil, Ocimum micranthum Willd., Labiatae in comparison with commercial essential oils, J Agric Food Chem 52 3486–3491 (2004)
- MK Khosla, Morphol. Study on the inter-relationship, phylogeny and evolutionary tendencies in genus Ocimum, J Plant Anat 6 93–106 (1995)
- BC Bennett and GT Prance, Introduced plants in the indigenous pharmacopoeia of Northern South America, Econ Bot 54 90–102 (2000)
- JF Morton, Atlas of medicinal plants of middle Americas Mahamas to Yucatan, Thomas: New York (1981)
- LC Di Stasi et al, Medicinal plants popularly used in the Brazilian tropical Atlantic forest, Fitoterapia 73 69–91 (2002)
- RE Schultes and R Raffauf, The Healing Forest, Dioscorides Press: Portland, OR (1990)
- R Vieira and F Simon, Chemical characterization of basil (Ocimum spp.) found in the markets and used in traditional medicine in Brazil, Econ Bot 54 207–216 (2000)
- K Helrich, Official Methods of Analysis of the Association of Official Analytical Chemists, 5th edn., AOAC International: Arlington, VA (1990)
- RP Adams, Identification of Essential Oil Components by Gas-Chromatography/Quadrupole Mass Spectrometry, Carol Stream, IL: Allured Publishing (2001)
- H Van del Dool and PD Kartz, A generalization of the retention index system including linear temperature programmed gas liquid partition chromatography, J Chromatogr 11 463-471 (1963)
- I Popov, G Lewin and R Baehr, Photochemiluminescent detection of antiradical activity, Assay of superoxide dismutase, Biomed Biochim Acta 46 775-779 (1987)
- I Popov and G Lewin, Photochemiluminescent detection of antiradical activity; IV: Testing of lipid-soluble antioxidants, J Biochem Biophys Methods 31 1-8 (1996)
- G Lewin and I Popov, Photochemiluminescent detection of antiradical activity III: A simple assay of ascorbate in blood plasma, J Biochem Biophys Methods 28 277-282 (1994)
- I Popov and G Lewin, Oxidants and antioxidants part B—Antioxidants homeostasis: Characterization by means of chemiluminescent technique, Methods in enzymology 300, 437-456 (1999)
- P Ziosi, E Besco, S Vertuani, F Bortolotti, G Baratto and S Manfredini, Determinazione della capacità antiossidante integrale (IAC) di formulazioni cosmetiche mediante fotochemiluminescenza (PCL), 30th Congresso Nazionale, Milano (2003)