Ultraviolet (UV) radiation from sunlight can cause many adverse effects in human skin—particularly in the 290–320 nm UVB range, which is the most harmful. Public concern about skin damage induced by sun exposure also has increased, leading to more specific regulatory requirements as well as controls for sun protection products. Evaluations for the level of UVB protection afforded by sunscreen products are based on in vivo methods.1, 2 These consist of comparing the UV radiation dose required for the appearance of a biological endpoint, in this case skin redness, with and without protection.
As in most other fields, industrial laboratories and health authorities require that such in vivo methods be substantiated by in vitro methods for ethical, economical and practical reasons. The determination of the in vitro SPF by means of a spectrophotometer was initially described by B.L. Diffey and J. Robson,3 then modified and improved to evaluate the level of UVB protection brought by the product. In vitro methods typically are based on the assessment of UV transmittance through a thin film of sunscreen sample spread on a roughened substrate. Many studies4–6 have been published in which the product quantity, spreading and substrate have been varied to improve the reproducibility and correlation of in vitro SPF evaluations with in vitro methods. Although repeatability can now be made relevant for very specific protocols, correlation with in vivo values is still a challenge for some products. This is clearly because a key parameter has not been considered: the affinity between the plate and the product, owing to the surface properties of the substrate.
The photo-efficiency of sunscreen products is mainly provided through intrinsic product absorbance and the quality of film-forming. The more homogeneous the film, the higher the SPF obtained. The quality of the film depends on different criteria such as spreading protocol and substrate surface properties, i.e., roughness and surface energy, for example. By changing the hydrophilic/hydrophobic surface characteristics, as described here, spreadability through wettability can be modified, impacting SPF.
Chemical approaches using white petrolatum or amphoteric surfactant have been studied;5, 7 nevertheless, it is logical to consider that no one single modification can solve the problem of poor affinity so long as it is product-dependent. Therefore, a new method is explored in this paper based on the step-by-step modification of interfacial properties through plasma treatments in order to improve the substrate/product affinity. Specifically, the aim of this study was to evaluate the influence of these stepped physical-chemistry modifications on in vitro SPF results, and consequently on in vivo/in vitro SPF correlation.
Materials and Methods
Plasma treatments currently are used by different industries, such as automotive, medical technology and electronics, to induce physical-chemistry modifications on materials, for example, to clean surfaces; for sterilization, surface activation and etching; and to provide a coating by plasma polymerization. These modifications improve the surface compatibility between two materials. Low-pressure plasma is created by means of a radio frequency (RF) oscillating electric field generated in the gas region of a chamber at a low pressure. The plasma thus consists of a mixture of mainly positively charged ions, highly reactive free radicals, electrons and neutral particles.
A low-pressure plasma systema was used for the described tests, which were carried out in a confined chamber having the interior dimensions of 280 mm length, 100 mm width and 100 mm height. The input power could be varied between 0 and 100 W with a 40 Khz RF, and pressure was reached by means of a vacuum rotary vane pump, 2.5 m3/hr.
Variations in process parameters such as pressure, power output, process time, gas flow and distance between electrode and sample can change the impact of the plasma treatment. Depending on the process gases and parameters, plasmas are capable of both mechanical work, through the ablative effect of kinetic transfer of electrons and ions with the surface, and chemical work, through the interaction of reactive radical species with the surface. In this study, to make such a process transposable to any laboratory, ambient air gas with about 21% oxygen was used and parameters were limited to process time and power output of the treatment. While the authors anticipated the substrate could be modified to improve compatibility with specific formulas, the specific modification required could not be anticipated, thus a set of modified substrates was proposed.
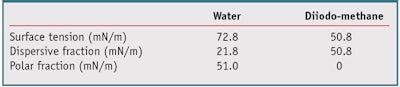
Surface free energy determination: The sessile drop method was used to determine the substrate surface energy and its polar and dispersive contributions. To characterize the polar and dispersive components, at least two liquids with known polar and dispersive fractions are required. For that, the contact angle of waterb and diiodo-methanec were measured by a drop shape analysis systemd by placing a 5-µL drop of each on the substrate; the characteristics of these liquids are listed in Table 1. For each surface energy measurement, nine contact angles—i.e., three plates and three spots per plate—were recorded for both water and diiodo-methane. The surface energy was then calculated by the Wu method;8 note that whereas J. van Oss9 and Owens and Wendt10 used the geometric mean of the surface tensions in their calculations, Wu uses the harmonic mean to mitigate the influence of large outliers and increase the influence of small values.
Substrate selection: A previous study6 showed the importance of substrate roughness on the reproducibility of in vitro SPF testing. Thus, molded polymethyl methacrylate (PMMA) platese with one face roughened and measuring 47 mm x 47 mm x 1.5 mm were used. The roughness parameter Ra, i.e., the mean arithmetic deviation of the assessed profile from the average profile, equaled 4.85 ± 0.29 µm. Surface topography parameters were controlled by the manufacturer, which processed the plates to meet the defined specifications.11
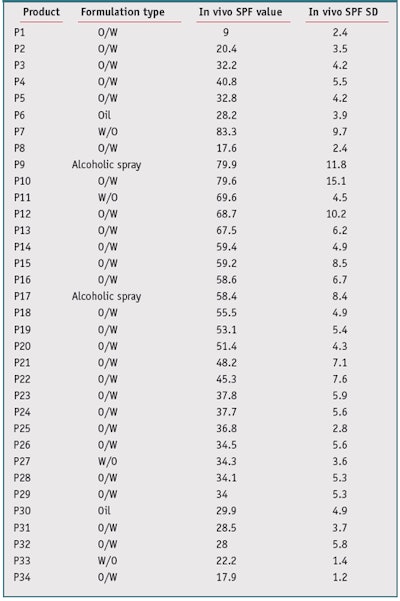
Sunscreen product selection: For this study, 34 sunscreen products covering various formulations were chosen. For all products, several measurements by different institutes had previously been taken, and in vivo SPF values ranged from 9 to 85 (see Table 2). These in vivo SPF values and standard deviations (SD) were determined according to the 2006 International Sun Protection Factor (SPF) Test Method, accepted by Cosmetics Europe (formerly Colipa), the Japan Cosmetic Industry Association (JCIA), the Personal Care Products Council (PCPC, formerly the Cosmetics, Toiletries and Fragrance Association or CTFA), and the South Africa CFTA (CTFA-SA).
Sunscreen application: Before application of the test products, each researcher’s application finger, without finger cot, was pre-saturated with the product. Nine 28.5 ± 0.5-mg drops of the test sunscreen product were applied by a 1-mL syringe across the whole PMMA plate surface. To ensure the correct application rate of 1.3 mg/cm², the syringe was weighed before and after product application. Immediately after weighing, the sunscreen product was spread over the whole surface using a defined sequence of circular, linear and light linear strokes. During spreading, application pressure was controlled by balance, movements by video, and temperature by a device that controls and maintains the temperature at the surface of the plate, 20°C in this study. After the product was spread, the sample was allowed to dry and settle for 15 min in the dark at room temperature, again at about 20°C. After drying, UV transmittance measurements were performed on the samples.
Transmittance measurements: Before taking any measurements, the transmittance analyzerf was validated and controlled by both specified standardsg and the linearity/additivity by calibrated reference standard Helioplate HD0 PMMA platesh to which UV filters were incorporated. Measurements were taken from the 290–400 nm range with 1 nm step. A PMMA plate covered with a film of white petrolatum, 15 mg applied, was used to obtain blank transmittance. Two different plates were used for each tested product and nine UV transmission spectras were recorded for each plate at the different application locations.
In vitro SPF calculation: By means of sunscreen UV transmittance measurement [T(λ)], the in vitro SPF could be calculated by combining the UV light attenuated by the sunscreen film, the erythema action spectrum, and a relevant solar emission spectrum according to Equation 1:
Eq. 1
where E(λ) is the relative effectiveness of UVR in producing erythema in human skin according to the erythema action spectrum (CIE-1987) at wavelength λ; I(λ) is the spectral irradiance received from the UV source at wavelength λ, in midday, midsummer sunlight; A(λ) is the monochromatic absorbance of the sunscreen layer at wavelength λ, with A(λ) = - log [T(λ)]; and d(λ) is the wavelength step, equal to 1 nm in this study. Finally, the in vitro SPF reported in this study is the arithmetical mean of the 18 in vitro calculated SPFn values for each n location. The range of variation of in vitro SPF values is characterized through standard deviation (SD) and coefficient of variation (CV%).
In vivo and in vitro SPF correlation: Two criteria were selected to determine the reliability of the in vitro SPF evaluations; first, the correlation between in vivo SPF values and in vitro SPF values through Person’s linear correlation coefficient r. The closer the correlation coefficient r is to 1, the more the in vitro SPF measurements correlate with in vivo measurements. Second, the linear regression was determined using the least square regression method, with zero as the fixed constant. Linear regression is used to show the tendency of in vitro SPF measurements to be higher or lower, compared with ideal correlations with in vivo measurements. Since the constant of the model was fixed, the correlation coefficient was calculated between values without adjustment.
Results and Discussion
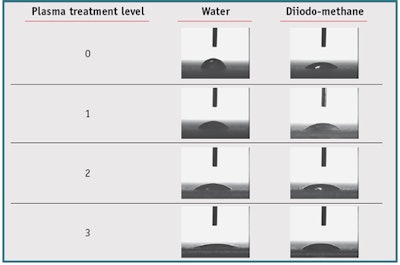
Plasma treatment parameters were adjusted to obtain different selected and correctly graduated levels of surface energy. To verify that these plasma treatment levels were correct, the contact angles of water and diiodo-methane drops were measured. From this data, the surface energy with dispersive and polar fractions was calculated. In total, three plasma treatment levels were fixed for this study. The detailed mean results on three plates are shown in Figures 1 and 2. Treatment duration did not exceed 10 min, including the time to reach process pressure, plasma treatment time, and venting. Images of water and diiodo-methane drops according to the level of plasma treatment are shown in Table 3. These results confirm that graduated plasma treatment modulates the substrate surface energy.
It also was observed that the impact of the plasma treatment on the substrate is more important on the polar contribution than the dispersive contribution. In addition to plates without plasma treatment, referred to as Level 0, with a surface energy of 50.32 ± 4.75 mN/m, the three levels selected for the study had surface energies of 63.98 ± 1.03 mN/m, for Level 1; 72.04 ± 1.19 mN/m for Level 2; and 80.53 ± 1.44 mN/m for Level 3 (see Figure 2). The mean standard deviation of the surface energy of all treatment levels did not exceed 2%, reflecting good repeatability of the process.
The stability of these surface energies generated by plasma treatments was observed for one week (see Figures 3 and 4), which confirmed that the treatments are not permanent (see Figure 4). According to the results, the plasma treatment evolved quickly on the first day. No changes in surface energy values were observed during the first hour after treatment; however, they then changed to lower values that were stable for up to one week. In comparison with values obtained at t0, the mean surface energy for all treatment levels decreased, after one day by 5.9%; after two days by 7.0%; and after one week by 8.2%.
In conclusion of the first step of this work, plasma treatment was shown to modulate the surface energy of the substrate including dispersive and polar fraction. It was also shown that surface energy was fairly stable during the first hour after plasma treatment, followed by a low degradation after a few hours and days, then stable for up to one week. Therefore, the system allows for a one-step combination plasma treatment/measurement within a short time period, i.e., no more than 10 min.
In vitro SPF Evaluation
Next, the in vitro SPFs for all 34 products were evaluated on substrates with and without the three plasma treatment levels. Detailed results are shown in Table 4, which also highlights (see shaded values) the best in vitro SPF measurements—i.e., lower GAP between in vivo and in vitro SPF values, among the four surface energy levels measured, as calculated by Equation 2:
Eq. 2 GAP = | in vivo SPF – in vitro SPF |
Different observations can be made. First, the in vitro spreading method with the same operator was repeatable with a low average coefficient of variation of 12.75%. Second, as expected, the choice of the most appropriate in vitro value related to the adapted plasma treatment level reduced GAP and depended upon the product. Overall, it is important to observe that in order to obtain in vitro SPF values that are closest to in vivo SPF values, although not necessarily equal, 26.5% of the products among the 34 selected needed no plasma treatment, 23.5% required a plasma treatment Level 1; 17.6%, a plasma treatment Level 2; and 32.4%, a plasma treatment Level 3. Thus, this demonstrates that only a single level of plasma treatment is not sufficient to improve in vivo/in vitro correlation. Also note that some in vitro SPF levels did not reach in vivo SPF values even if a plasma treatment was applied on the substrate. Obviously, adaptations other than interface adjustments should be required to solve the issue of poor correlation in the case of these products.
In conclusion of this second part of the work, it was shown that plasma treatment has a significant impact on product spreading, and consequently on the in vitro SPF measurement. The choice of a specific plasma treatment to improve in vivo and in vitro correlation is product-dependent, and allows for matching the in vivo SPF value for a majority of the tested products.
In vivo/In vitro SPF Correlation
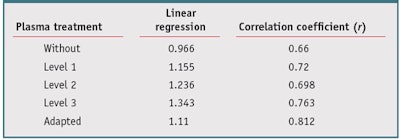
From data presented in Table 4, the in vivo/in vitro SPF correlation was compared with and without different plasma treatment levels. Results shown in Figure 5, without the plasma treatment, led to a correlation coefficient of 0.660, which indicates a moderate correlation with in vivo SPF. However, resulting linear regression follows the equation y = 0.966x, due to some in vitro SPF values being very high. So in general, in vitro SPF values were underestimated. Compared with the results without plasma treatment, a plasma treatment Level 1 (see Figure 6) allows a slightly better correlation coefficient, r = 0.720, and a linear regression of y = 1.155x. Data with plasma treatment Level 2 (see Figure 7), with a correlation coefficient r = 0.698, was better than no plasma treatment but slightly worse than Level 1. The linear regression was y = 1.236x. From results obtained with a plasma treatment Level 3 (see Figure 8), the correlation coefficient was improved and reached 0.763 with a linear regression of y = 1.343x. In this case, the correlation coefficient was better than other levels but in vitro SPFs were generally overestimated. Whatever the level of plasma treatment used, the in vivo/in vitro SPF correlation coefficient was improved compared to no treatment, particularly with plasma treatment Level 3.
The authors next also examined whether an adapted plasma treatment could improve the in vivo/in vitro SPF correlation. For that, the adapted plasma treatment was chosen for each product based on the results previously obtained for different levels of plasma treatment, to reduce GAP (see Equation 2) between in vivo and in vitro SPF values. Clearly, in this first step, the choice was based on the observation of the known in vivo SPF. This selection should be made using a predictive method to determine the level of required modification for each product. Whatever the treatment level, the adapted plasma treatment (see Figure 9) allows a better correlation coefficient of r = 0.812, compared with the correlation obtained from values issued from untreated substrate. Linear regression follows the equation y = 1.110x. Detailed results of linear regression and correlation coefficient were summarized in Table 5.
It is therefore possible to conclude that in vitro SPF values were very close to in vivo values for the majority of the products, except for the few that were very overestimated (SPF > 100). Thus, it is possible to find, and likely to define, a plasma treatment level to allow for an in vitro SPF measurement closer to the in vivo value.
In conclusion of this portion of the study, it was shown that with the adapted plasma treatment, an in vitro SPF result closer to the in vivo SPF value with a better coefficient correlation (r = 0.812) was obtained, in comparison with no plasma treatment (r = 0.660) or a single plasma treatment level. Again, the same surface energy treatment, at any level, was not suitable for the whole set of products, so the plasma treatment must be adapted to increase in vivo/ in vitro SPF correlation.
Conclusion
Overall, from the various experiments performed in this study, it appears that plasma treatment allows for the scaling of different surface energy levels for PMMA substrates. On a large set of products, it was demonstrated that substrate surface energy is an important criterion to improve in vivo/in vitro SPF correlation. Furthermore, the plasma modification produces a reliable, repeatable treatment method.
The required level also depended upon the product. Indeed, with an adapted plasma treatment chosen between three levels of treatment and without treatment, the correlation coefficient raised to 0.812, whereas without treatment it was about 0.660. Thus, no single plasma treatment level was suitable for any product to improve in vivo/in vitro SPF correlation. Since the in vivo SPF should not be known when evaluating the in vitro value, the next steps in this process will be to define an eligibility criterion to select the plasma treatment level required to predict in vivo SPF, and to identify parameters that could explain unsatisfactory results. These two aspects will be treated in future work.
Acknowledgements: The authors wish to thank Dominique Moyal, of L’Oréal R&I, for her support.
References
Send e-mail to [email protected].
- US FDA, Department of Health and Human Services, Sunscreen drug products for over-the- counter human use, Final Rule 21 CFR parts 201 and 310, Federal Register 76 (117) (Jun 17, 2011)
- ISO 24444: Cosmetics—Sun protection test method—In vivo determination of the sun protection factor (SPF) (2010)
- BL Diffey and J Robson, A new substrate to measure sunscreen protection factors throughout the ultraviolet spectrum, J Soc Cosmet Chem 40 127–133 (1989)
- M Pissavini et al, Determination of the in vitro SPF, Cosm & Toil 118(10) 63–72 (Oct 2003)
- L Fageon, D Moyal, J Coutet and D Candau, Importance of sunscreen products spreading protocol and substrate roughness for in vitro sun protection factor assessment, Intl J Cosmet Sci 1–13 (2009)
- L Ferrero, M Pissavini, A Dehais, S Marguerie and L Zastrow, Importance of substrate roughness for in vitro sun protection assessment, IFSCC 9(2) 1–13 (Apr/Jun 2006)
- S Marguerie, M Pissavini, A Baud, T Carayol and O Doucet, A new chemical approach to optimize the in vitro SPF method on the HD6 PMMA plate, J Cosmet Sci 63 243–254 (Jul/Aug 2012)
- S Wu, Polar and nonpolar interactions in adhesion, J Adhesion 5 39–55 (1973)
- DK Owens and RC Wendt, Estimation of the surface free energy of polymers, J Applied Polymer Science 13(8) 1741–1747 (1969)
- CJ Van Oss, Interfacial forces in aqueous media, Marcel Dekker Inc, NY (1994)
- M Pissavini, S Marguerie, A Dehais, L Ferrero and L Zastrow, Characterizing roughness: A new substrate to measure SPF, Cosm & Toil 124(9) 56–64 (Sep 2009)

!['[Sunscreen] developers will be able to innovate more efficiently while maintaining high standards of quality and safety for consumers.'](https://img.cosmeticsandtoiletries.com/files/base/allured/all/image/2024/06/woman_outside_using_sunscreen_on_face_ISO_test_standards_AdobeStock_783608310.66678a92029d9.png?auto=format%2Ccompress&fit=crop&h=191&q=70&rect=62%2C0%2C2135%2C1200&w=340)









