*Adapted with permission from M Rule, K Saliesh, T Haw-Yueh, H Zhai and HI Maibach, Percutaneous Penetration Enhancers: An Overview, in Handbook of Cosmetic Science and Technology, 4th edn, CRC Press, Boca Raton, FL (2014) pp 141-156
The first seminar on in vivo human skin penetration was presented to a major international skin care company in 1972—and at the end of the talk, the convening scientist stated (quietly) that this information was irrelevant since cosmetic ingredients do not penetrate.
In the following decades, much has changed; it is now generally accepted that many or most low molecular weight cosmetic ingredients penetrate skin. As such, today’s questions revolve more around delivery mechanisms, quantification and interpretation.1
This two-part column describes penetration enhancers for the active (physical) and passive (chemical) delivery of chemicals through the skin. The present installment serves as an introduction to active methods such as iontophoresis—a technique that dates back to in vivo rabbit data in the 19th century. Several products based on similar mechanisms have been theorized, patented and commercialized, although none has achieved the same significance or clinical presence as passively diffused transdermal drugs. Clearly, physical delivery works, so it remains to be seen why the industry has yet to fully embrace it.
The second installment in this series will highlight passive delivery via chemicals; i.e., so-called penetration enhancers.
Physical Penetration Enhancement
Physical means of penetration enhancement mainly incorporate methods that transiently circumvent the normal barrier function of the stratum corneum (SC). Although their mechanisms are different, these approaches share the common goal of disrupting the SC structure to create breaches that are significant enough for macromolecules to permeate. Two well-known examples are the aforementioned iontophoresis and sonophoresis; others include microneedling, thermal ablation and electroporation.
Physical delivery works, so it remains to be seen why the industry has yet to fully embrace it.
Energized delivery: Iontophoresis uses a small electrical charge to noninvasively propel charged particles of a desired active into the skin. Sonophoresis, on the other hand, uses low-frequency ultrasound to increase the permeability of skin, allowing agents of interest to penetrate. The breaches created by these methods are on the order of nanometers, permitting the transport of only small drugs.2
Microneedling: A newer technology for macromolecule delivery is microneedling. This system uses an array of tiny, needle-like structures to create transport pathways into skin on the order of microns, permitting the transport of macromolecules and, potentially, supramolecular complexes and microparticles. Such systems have enhanced the penetration of macromolecules many-fold,3 while also offering painless drug delivery4, 5 since they directly intervene with skin but do not penetrate significantly enough to excite nerve endings.6
Thermal ablation: Thermal ablation in short, controlled blasts, is another approach to create micro-channels in skin. Heat increases the permeability of skin by disordering the lipid structure and keratin network, and even decomposing/vaporizing keratin to create micron-scale holes, allowing actives of interest to penetrate.
Electroporation: Similar to iontophoresis, electroporation uses small pulses of high energy electricity to open skin channels. When an electrical field is applied to cells, it increases the permeability of the cell membrane, allowing actives of interest to enter.
Enhancing Effects
The physical skin penetration methods discussed also offer alternatives to oral and injectable drug delivery. Furthermore, combining then with chemical penetration enhancers (CPEs, to be discussed in part II of this series) can increase the dispersion of CPEs and the penetration of actives.
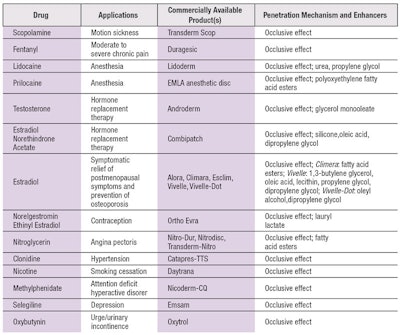
Interestingly, despite the plethora of CPE and physical delivery options, most of today's transdermal drug delivery (TDD) products adopt skin occlusion as the primary mechanism for penetration enhancement. Perhaps this is due to its simplicity and convenience. Table 1 summarizes current technologies used for the physical penetration enhancement of drugs.
Conclusion, Part I
Taken together, years of experience in delivering drugs and actives through the skin, mainly via chemical routes, could pave the way for delivering cosmetic ingredients via both physical and chemical delivery systems, or combinations thereof.
References
- H I. Maibach and M A. Ngo, 15 Factors of Percutaneous Penetration of Pesticides, in Parameters for Pesticide QSAR and PBPK/PD Models for Human Risk Assessment, American Chemical Society (2012) pp 67–86
- MR Prausnitz, Microneedles for transdermal drug delivery, Adv Drug Deliv Rev 56(5) 581–587 (2004)
- BW Barry, Novel mechanisms and devices to enable successful transdermal drug delivery, Eur JPharm Sci 14(2)
101–104 (2001) - S Kaushik, AH Hord, DD Denson, et al., Lack of pain associated with microfabricated microneedles, Anesth Analg 92(2) 502–504 (2001)
- RK Sivamani, B Stoeber, GC Wu, et al., Clinical microneedle injection of methyl nicotinate: stratum corneum penetration, Skin Res Technol 11 152–156 (2005)
- K Paudel et al., Challenges and opportunities in dermal/transdermal delivery, Ther Deliv 1(1) 109–31 (2010)