This is the fourth of a four-part series on SLNs and NLCs (see Part I, Part II and Part III).
Percutaneous absorption is strongly dependent upon the physicochemical properties of the active ingredient applied to skin—i.e., molecular weight (MW), liposolubility and octanol/water partition coefficient—and on the type and properties of the delivering vehicle. When this vehicle is based on lipid nanoparticulates, enhanced permeation has been reported. SLNs and NLCs are typical nanoparticulate carriers composed of physiological and biodegradable lipids. These carriers were reviewed in Parts I, II and III of this series, with respect to chemical content, production techniques and physicochemical characterization. The present installment, Part IV, discusses the percutaneous absorption of actives loaded into SLNs and NLCs and their formulation in dermal cosmetics. This series will be expanded to include an additional Part V, which will focus on the rheological and texture analysis of lipid nanoparticles.
Partitioning and Diffusion
The nature of a carrier vehicle is known to affect the depth of skin penetration of actives therein, owing to its role in solubility and release as well as its influence on transepidermal water loss. In parallel with in vitro and in vivo permeation studies, the thermo- dynamic activity of the active ingredient, i.e. the rate of transfer between the vehicle and the skin, must be assessed. More importantly than the concentration of active in a formulation, skin influx must be determined with respect to octanol/water partition coefficient, Km, which is the active ingredient distribution between a membrane, m, at equilibrium.
When an active is loaded in lipid matrices such as SLNs or NLCs, which are then dispersed in a semi-solid vehicle, e.g., o/w creams or hydrogels, the stratum corneum is the first layer with which it comes into contact. To be absorbed into the stratum corneum, the active must be released from the lipid matrix, diffuse in the vehicle, and reach the outermost layer of skin. Thus, the first critical step in determining the skin absorption level of the active is the partitioning between the particles and vehicle, and between the vehicle and the skin.
Partitioning between the vehicle and skin can be expressed as:
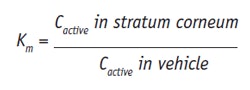
Eq. 1
where C refers to concentration. From this equation it can be seen that when the active has a higher affinity to the stratum corneum than to the vehicle, Km increases. Since lipophilic actives have greater affinity to the stratum corneum, these tend to accumulate in this layer. For in vitro experiments, the octanol/water partition coefficient, Ko/w, is commonly used. For less lipophilic actives, to enhance their partitioning into the stratum corneum, the most common approach is to change their solubility in the vehicle, for instance by formulating them in SLNs or NLCs. Skin permeation of actives is thus influenced by partitioning and diffusion, and these parameters can be correlated in the permeability coefficient, P, measured in moles/sec (m/s), given as:
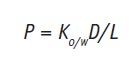
Eq. 2
where D stands for the diffusion coefficient (m2/s), and L for the length of the diffusion pathway of the active. The L value, however, is difficult to estimate because the exact diffusion pathway of an active into the skin cannot be determined; absorption can occur through intercellular or transcellular pathways.
Taking into account the Ko/w value and the relative molecular weight (MW) of the active, it is possible to estimate the value of log P as follows:1
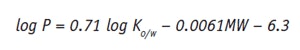
Eq. 3
This value gives the formulator an idea of the lipophilicity of the active ingredient. Actives with lower log P values usually have lower mean permeation rates. Higher permeation rates are typically associated with log P values above 0.5.
The diffusion of the active through the skin is described by Fick’s First Law, expressed as:
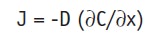
Eq. 4
where J is the flux, given in mass/m2/sec, i.e., the rate of transfer per unit area at a given time and position; ∂C is the differential concentration change; and ∂x is the differential distance. Since J is directly related to a decrease in thermodynamic activity, it is given as negative value. Assuming that the active freely diffuses within the skin—i.e., is not metabolized, degraded or bound, there is a differential mass balance, described by Fick’s Second Law:
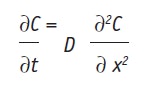
Eq. 5
where J is the constant, at the steady state, which can be given as:
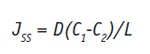
Eq. 6
where C1 and C2 are the active mass concentrations, given in kg/m3, in the skin between the membrane at x = 0 and I. Modeling the dissolution rate of an active in vitro, under sink conditions, i.e. when C2 = 0, Jss becomes:
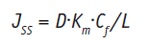
Eq. 7
Combining this equation with Equation 1, the dermal permeability coefficient, P (m/s), can be given as:
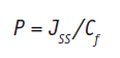
Eq. 8
There is a close relationship between permeation enhancement and lipid bilayer fluidization in that the lipid lamella of the stratum corneum is the main site of action. The diffusion coefficient of an active (Jss) is thus influenced by the disordering or fluidization of the lipid structure of the stratum corneum.2
It has already been reported that nanoparticles do not penetrate intracellularly or between corneocytes.3 However, depending on the lipid composition of the matrix, SLNs and NLCs can mix with the lipids of the sebum and decrease the skin’s resistance to polar actives, acting as penetration enhancers without any chemical binding or disruption of the corneocytes. In addition, the particles can deliver actives into the hair follicles.4, 5
While the stratum corneum is characterized by a semi-solid diffusive structure, the dermis and viable epidermis are extensively hydrated and their diffusion coefficients are typical of liquid states. The diffusional resistance of the stratum corneum to water is approximately 1,000 times that of either the viable epidermis or the superficial region of the dermis. In this latter layer, molecules probably move within waterfilled interstices. Hydrophilic actives will thus have smaller diffusion coefficients than the water, and the stratum corneum will become even more dominant in the total permeation process. Actives with log Ko/w values between 1 and 4 favor dermal absorption and thus increase the risk of systemic effects, particularly if the substance is more lipid-soluble than water-soluble. For those actives with log Ko/w values below 0, penetration into the stratum corneum is limited by their hydrophilicity, so to effectively overcome the stratum corneum’s barrier and deliver actives to the skin, semisolid personal care products formulated with SLNs and NLCs have been proposed.
Formulating Cosmetics With SLNs and NLCs
Dermal cosmetics are used for many purposes but mainly as sunscreen formulations,6 for anti-aging benefits,7 and to increase hydration and elasticity.8, 10 Several such actives, e.g., UV filters, co-enzyme Q10 and retinoids, have been formulated in both conventional formulations and colloidal systems. Conventional formulations have reported problems with low uptake rates due to the barrier function of the stratum corneum, as previously described, and pose the risk of systemic absorption, which can lead to unwanted side effects.8, 11 These problems can be avoided by improving the delivery of actives specifically to the target site via colloidal delivery systems such as SLNs and NLCs.11, 12
SLNs and NLCs have gained widespread interest in recent years as drug carriers for dermatological cosmetic purposes, for better permeation or prolonged action of the active on the skin, or to directly target specific skin layers.10, 13 Recently, research devoted to SLNs and NLCs has gradually increased in the field of cosmetics, incorporating active ingredients such as retinol (vitamin A),14, 15 tocopherol acetate (vitamin E),16, 17 ascorbyl palmitate (vitamin C),18–20 coenzyme Q10,7, 9 titanium dioxide21 and octyl-dimethylaminobenzoate.22 Comparing the use of semisolid vehicles for dermal cosmetics, Jenning and Shafer-Korting14 produced SLNs loaded with vitamin A in hydrogel and in an o/w cream and tested the influence of the carrier on the penetration of vitamin A into porcine skin. High concentration values were found in the upper skin layers but due to the active expulsion from the carrier, this localizing action appeared to be limited to 6–24 hr, in the case of hydrogel. Best results were obtained with vitamin A SLNs incorporated in the o/w cream, which showed delayed expulsion or release from the system.
Upon topical application, SLNs and NLCs create an occlusive film on the skin, leading to an increase in skin hydration followed by the enhanced permeation of active ingredients.23 In addition, since SLNs may be formulated with higher emulsifier concentrations, they can contribute to the deeper permeation of actives, in comparison with NLCs. Küchler et al. developed dendritic core-multishell (CMS) nanoparticles (mean size = 20–30 nm) loaded with Nile Red and compared their penetration into pig skin with that of SLNs (mean size = 150–170 nm) and a reference cream.24 SLNs showed a penetration enhancement of 3.8-fold in the stratum corneum and 6.3-fold in the epidermis, compared with the reference cream; CMSs showed an increased penetration of 8-fold in the stratum corneum and 13-fold in the epidermis. The differences between SLNs and CMSs were attributed to the character of the nanoparticles and not to the smaller size of the CMSs, showing an enhanced risk of toxicity due to the irreversible effects they have in the skin.
Production of Semisolid Vehicles
Semisolid vehicles are employed in topical products to deliver actives to the skin or mucosa to provide localized or systemic effects at the application site. Semisolid vehicles flow or deform when an external force is applied.25 Thus, to maintain consistency for topical application, SLNs and NLCs must be e.g., hydrophilic gels,14, 26 o/w creams,7, 27 or on hydrophobic vehicles,28 to allow for more comfortable application and easy spreading—since semisolid vehicles with a high consistency are more difficult to spread.29
The incorporation of SLNs and NLCs into dermal formulations is a simple procedure but requires two production steps, the first of which is the necessary mixing of the nanoparticle suspension with the semisolid vehicle by gentle stirring.30 This can cause issues such as incompatibilities with the ingredients of the dermal vehicles, resulting in possible aggregation or dissolution of the SLNs and NLCs in the vehicles.31
SLNs/NLCs in Hydrophobic Vehicles
Hydrophobic bases such as paraffin, vegetable oils and fatty alcohols can be used to disperse lipid nanoparticles to form w/o systems32, 33 with a high degree of occlusivity. Provided that SLNs and NLCs are not soluble in the hydrophobic bases,33 the penetration of the active ingredient incorporated into the lipid matrices will be reduced in comparison with o/w systems. Therefore, moisture vapor transmission occurs faster from w/o systems than from the hydrophobic bases alone.34 The main disadvantage of using hydrophobic vehicles is that residues remaining on the skin will not easily be removed with water alone. On the other hand, an advantage is the reduction of their fatty character with sufficient occlusion and without the intensive penetration of active ingredients.33
Another problem with the incorporation of SLNs and NLCs in hydrophobic vehicles is the risk of solubilizing these particles with the hydrophobic vehicle. Therefore, screening studies of lipid raw materials must be a selection criterion for the qualitative composition of the final formulation. During these pre-formulation studies, DSC analysis of lipid solid mixtures is an important tool. Due to their character, w/o vehicles seem suitable to carry inorganic filters for sunscreen purposes; lipophilic aqueous emulsions (w/o) are preferred due to their water-resistant character.
SLNs/NLCs in O/W Creams
Creams are biphasic systems produced by dispersing an inner oil phase in an external water phase, following cooling to about 30°C. These vehicles are the most frequently employed for the delivery of cosmetic ingredients.35 They are also preferred for topical photoprotection due to their suitable viscosities, which can be limited to small surface areas, e.g., the face, or adapted to large areas, e.g., the whole body. Also referred to as milks, they can provide a wide range of SPF values, up to 50+, and enable various combinations of both hydrophilic and lipophilic filters to which zinc oxide and titanium dioxide can be added.
In such formulations, SLNs or NLCs are added to the vehicle by replacing a part of the water phase with a concentrated SLN or NLC dispersion. To match the lipid content of the original cream, it is therefore necessary to use less of the initial lipid amount since the inclusion of the lipid nanoparticles achieves the final lipid content.12 After production of the cream, the concentrated dispersion of SLNs/NLCs is mixed by stirring at ≥ 30°C or room temperature. This process prevents the nanoparticles from melting, which can lead to an undesired change of the internal particle structure.36
Gulbake et al.37 studied oxybenzoneloaded SLNs to improve the effectiveness of a sunscreen by incorporating them into an o/w cream base and comparing it both in vitro and in vivo with a similar drug-free formulation. The cream base formulation containing 5% oxybenzone-loaded SLNs showed a slower drug release and better sun protection factor (25+), compared with the SLN-free cream. Oxybenzone-loaded SLNs also showed prolonged release in the stratum corneum.
SLNs/NLCs in Hydrophilic Gels
Hydrophilic gels can be classified either as natural, e.g., those based on chitosan, dextran and xanthan gum; semi-synthetic, such as gels from cellulose derivatives including hydroxypropyl or carboxymethyl cellulose; or synthetic, i.e., those based on polymers. Synthetic and semi-synthetic gels have the advantage of time-proven safety in topical uses, as well as low price and ease of preparation.29 The incorporation of SLNs and NLCs into hydrophilic gels provides the added advantages of low toxicity, unique physical properties, biocompatibility and muco-adhesiveness.25, 33 Moreover, whereas creams are traditionally employed to solubilize hydrophobic active ingredients, poorly water-soluble compounds can instead be loaded into aqueous topical gels by encapsulation within nanoparticles; such gels are generally preferred over creams, in terms of cosmetic acceptability.38, 39
Souto et al.25 evaluated SLN and NLC aqueous dispersions before and after their incorporation into different types of hydrophilic gels including xanthan gum, hydroxyethylcellulose (4000 cP) and carbomera (and) chitosan. Results showed that these systems are complex and their performance is dependent upon the structure of the gel-forming polymer used for hydrogel preparation. Increasing the solid lipid content of the dispersed phase increased the elastic component. Moreover, both carriers showed good physical stability after their dispersion into semisolid vehicles. In another study, Bhalekar et al.40 produced miconazole-loaded SLNs in hydrophilic gels produced with carbomerb. Ex vivo results showed the gel contributed a higher localization of miconazole nitrate in the skin, compared with conventional gel.
SLN/NLC-based Creams
For topical application, the nanoparticles alone can be produced in a one-step process using high lipid concentrations.28 These lipid nanoparticles may be added to vehicle creams, lotions or hydrogels12 as they are formulated or post-production. If during formulation, a part of the water in the vehicle is replaced by a highly concentrated dispersion of nanoparticles, which are sufficiently stabilized to avoid their coalescing with the oil droplets of the vehicle. If the production process occurs at a temperature higher than the melting point of the lipid nanoparticles, they will melt but will recrystallize during cooling at the end of the process.36
Lippacher et al.28 studied the production of SLN dispersions with a semisolid consistency produced by one-step high pressure homogenization. The authors used a high concentration of cetyl palmitate stabilized with sucrose palmitate. Results showed the newly developed semisolid SLN dispersions had the same consistency as SLN dispersions in semisolid carriers and advantages such as: high SLN loading; a timesaving, one-step production method; less ingredients; no incompatibilities with other excipients; and easy scale up.
Drugs or cosmetic actives can be delivered entrapped in lipid nanoparticles, dissolved in the vehicle, or both. If the drug or cosmetic active is present in both the lipid particles and the vehicle, a supersaturated system is formed. Similar systems can be developed where drug-loaded lipid nanoparticles are dispersed into semisolid bases already saturated with the drug. During storage, the drug remains in the lipid matrix because these particles preserve their polymorphic form, which is typical for lipids. After application onto skin, the drug or active ingredient is expelled due to the increase in temperature and water loss, which transforms the lipid matrix into a more ordered structure and expels the drug or active from the lipid matrix to semisolid bases already saturated, leading to drug or active ingredient penetration.36
The technological aspects of lipid nanoparticle formulas designed for the pharmaceutical versus cosmetic fields are similar; i.e., the incorporation of actives into particles, the incorporation of particles into vehicles, their stability in the vehicle, etc. However, the time for product development and market introduction for cosmetic products is much shorter due to more complex regulations for pharmaceutical products.41
Marketed Formulations
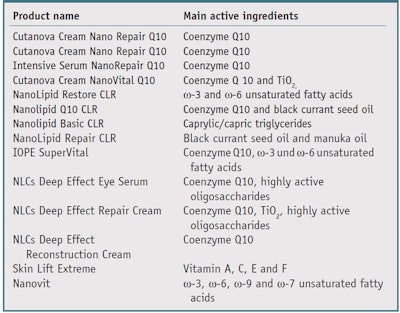
Currently, SLNs and NLCs are commercially available from several pharma cosmetic companies. Table 1 shows the product name and main active ingredients, figuring in the colloidal system.
Lipid nanoparticles with CoQ10: Coenzyme Q10 (CoQ10) is a hydrophobic molecule42 that acts as a cofactor in the mitochondrial respiratory chain, where it transfers free electrons from complexes I and II to complex III during oxidative phosphorylation and ATP synthesis.7 CoQ10 protects membrane phospholipids and proteins from lipid peroxidation by scavenging free radicals directly and/or by increasing levels of regenerated tocopherol.43, 44
During the aging process CoQ10 synthesis is reduced, leading to lower plasma levels and tissue concentrations in elderly individuals.45 This lessened efficiency in the antioxidant system has been proposed as a factor in skin aging and for this purpose, the cosmetic industry has been employing CoQ10 in anti-aging products.46 CoQ10 is a potential preventive medication against skin photoaging47, 48 and an antioxidant with tenfold higher levels in the epidermis than in the dermis.42, 49 The main disadvantage of this active is its poor water solubility,50, 51 which limits its bioavailability and topical application.50
The encapsulation and permeation of CoQ10-loaded NLCs were studied by Yue et al.47 CoQ10-loaded NLCs were characterized for size by freeze-fracture transmission electron microscopy, and viability tests on fibroblasts were undertaken with CoQ10-loaded NLCs, CoQ10 in emulsion, and a control. The results show that nanoparticles were homogenous, with a droplet size of approximately 65 nm. Figure 1 demonstrates that the protection of CoQ10-loaded NLCs was more effective than CoQ10 in an emulsion. In addition, CoQ10-loaded NLCs significantly enhanced the antioxidative capacity of fibroblasts in which oxidative stress was induced by UVA irradiation. CoQ10-loaded NLCs also improved skin penetration in vitro and in vivo, compared with a non-particulate formulation in the same concentration. Considering the advantages of CoQ10-loaded NLCs in protecting fibroblasts, as well as their strong penetration ability, this formulation is reported to be suitable for topical drug delivery.
Junyaprasert et al.8 studied CoQ10- loaded NLCs incorporated in the hydrophilic vehicle xantham gum to investigate the effects, on long-term physical and chemical stability, of increased oil loading in NLCs using cetyl palmitate and medium chain triacylglycerols. The CoQ10-loaded NLCs demonstrated good long-term physical and chemical stability, and in vitro permeation studies showed the amount of CoQ10 in the skin was affected by the amount of oil content in the NLCs. The deeper penetration of CoQ10 was attributed to the higher amount of active released from the particles when using higher oil content in NLCs, in addition to their known occlusive effect on the skin.
In relation, Pardeike et al.7 analyzed the first NLC-containing cream available on the cosmetic marketc for its tolerability and irritancy on skin. It was compared with an identical o/w cream without NLCs but with similar physical properties to determine its influence on skin hydration and applicability. Results showed higher levels of hydration and CoQ10 in the stratum corneum from the NLC CoQ10 cream, compared with the negative control cream.
Dingler et al.,17 Müller et al.,35 Teeranachaideekul et al.52 and Souto et al.25, 53 previously reported the ability of an SLN-containing o/w cream to increase skin hydration significantly more than a conventional o/w cream without SLNs. The results obtained for the first generation of lipid nanoparticles could be confirmed for the second generation of lipid nanoparticles.7, 8
Lipid nanoparticles with ω-3 unsaturated fatty acids: Omega-3 (ω-3) and ω-6 unsaturated acids are known to play an important role in living organisms, as well as for the proper functioning of skin54—specifically for anti-inflammatory55 and UV protection,56 thus preventing and treating processes involved in skin aging. Amore Pacific of South Korea also reported the beneficial effects of these molecules on skin, which became the basis for one of its brands incorporating black currant seed oil (BCO). Since this oil is not particularly stable, stress tests were undertaken on creams containing the oil with and without BCO-loaded NLCs. Performed at 48°C, the tests exposed the samples to pure oxygen, and the effect was quantified by the determination of the peroxide number. Results found that NLCs were able to protect and slow the oxidation process of the oil.57 The products with BCOloaded NLCs were introduced to the market in September 2006 as a cream, eye cream and serum. In relation, based on this data and experiences reported by Hawaiian women, who historically have used kukui oil to care for the skin of their babies and infants, products with kukui oil-loaded NLCs were also developed and placed on the market.58
Lipid nanoparticles with retinol: Much has been learned in the past decade about the action of retinol and its derivatives—i.e., retinoids or vitamin A derivatives, on the regulation of epithelial cell growth, differentiation, sebum production, collagen and especially wrinkles59, 60 thanks to the increased popularity of these actives in dermatological and cosmetic applications. However, they also show adverse side effects such as local irritation, erythema, peeling, burning and increased susceptibility to sunlight.61, 62 In addition, retinol has very low water solubility and high instability in the presence of air, light and heat, limiting its formulation in conventional skin care products.
To overcome these drawbacks, the active can be loaded into SLNs and NLCs and incorporated into hydrogels or o/w creams. Pople and Singh produced hydrophilic vehicles, e.g., carbomers and xanthan gum, enriched with vitamin A-loaded SLNs;63 in vitro penetration studies showed an almost twofold higher concentration of the active on skin when applied via hydrogels containing vitamin A-loaded SLNs. This was in comparison with conventional hydrogels formulated with vitamin A alone. In relation, in vivo skin hydration studies in albino rats revealed improved skin hydration with vitamin A-loaded SLNs without any reports of skin irritation, erythema or edema.
Carlotti et al. prepared retinyl palmitate-loaded SLNs dispersed in o/w emulsions and evaluated the protective effects of the particles on the photochemical degradation of the active.64 Results showed the particles improved the photostability of retinyl exposure, compared with an o/w emulsion. In another recent study, alltrans retinoic acid-loaded SLNs were produced and their comedolytic effects were evaluated in vivo in comparison with a conventional marketed formulation.65 The thickness of the epidermis and stratum granulosum were found to increase with both formulations, suggesting that the encapsulation of the active in SLNs preserved its epidermal effects after topical application. The authors also compared the thickness of the epidermis granular layer, which was greater for skin treated with the SLN-containing formulation than for the marketed formulation. From horizontal sections of skin biopsies, the authors also reported a significant reduction in utricle size when the active was loaded in nanoparticles. Further, the cumulative irritation index was higher for the marketed formulation than for the SLN-containing formulation. These results are promising in terms of developing innovative topical anti-aging and anti-acne formulations based on retinoids.
Conclusion
The stratum corneum is the outermost barrier that affects the penetration of active ingredients, such as from dermal cosmetics, as well as other numerous formulation parameters. While in some cases penetration is enhanced—e.g., in the case of lipophilic actives, in others, diffusion becomes a limiting step—e.g., hydrophilic molecules. To overcome these limitations, SLNs and NLCs can optimize topical delivery when formulated into semisolids. The technology is simple, reproducible and scalable, and successful examples in the market already exist, mainly for anti-aging cosmetics.
In addition, SLN and NLC dispersions show rheological behavior appropriate for skin applications, which can be verified through a critical selection of tests to evaluate the viscoelastic and texture properties of the final product. These tests can ensure consumer acceptance and successful marketing, thus Part V of this series, scheduled for the October 2012 issue, will focus on analyzing the rheology and texture of lipid nanoparticles and their promising results to shape the future of safe personal care.
References
- PO Potts and RH Guy, Predicting skin permeability, Pharm Res 9(5) 663–669 (May 1992)
- R Mendelsohn, CR Flach and DJ Moore, Determination of molecular conformation and permeation in skin via IR spectroscopy, microscopy and imaging, Biochim Biophys Acta 1758(7) 923–933 (Jul 2006)
- GJ Nohynek, EK Dufour and MS Roberts, Nanotechnology, cosmetics and the skin: Is there a health risk? Skin Pharmacol Physiol 21(3) 136–149 (2008)
- H Chen et al, Podophyllotoxin-loaded solid lipid nanoparticles for epidermal targeting, J Control Release 110(2) 296–306 (Jan 10, 2006)
- U Munster et al, RU 58841-myristate—Prodrug development for topical treatment of acne and androgenetic alopecia, Pharmazie 60(1) 8–12 (Jan 2005)
- I Lacatusu, N Badea, A Murariu, D Bojin and A Meghea, Effect of UV sunscreens loaded in solid lipid nanoparticles: A combinated SPF assay and photostability, Molecular Crystals and Liquid Crystals 523 247–259 (2010)
- J Pardeike, K Schwabe and RH Müller, Influence of nanostructured lipid carriers (NLC) on the physical properties of the Cutanova Nanorepair Q10 cream and the in vivo skin hydration effect, Intl J Pharmaceutics 396(1–2) 166–173 (2010)
- VB Junyaprasert, V Teeranachaideekul, EB Souto, P Boonme and RH Müller, Q(10)-loaded NLC versus nanoemulsions: Stability, rheology and in vitro skin permeation, Intl J Pharmaceutics 377(1–2) 207–214 (2009)
- SA Wissing and RH Müller, The influence of solid lipid nanoparticles on skin hydration and viscoelasticity—In vivo study, European J Pharmaceutics and Biopharmaceutics 56(1) 67–72 (2003)
- JW Wiechers and EB Souto, Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs) as novel delivery systems for cosmetic actives, part 1, Cosm & Toil 125(10) 22–30 (2010)
- MJ Choi and HI Maibach, Liposomes and niosomes as topical drug delivery systems, Skin Pharmacology and Physiology 18(5) 209–219 (2005)
- RH Müller, M Radtke and SA Wissing, Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations, Advanced Drug Delivery Reviews 54 S131–S155 (2002)
- W Weyenberg et al, Cytotoxicity of submicron emulsions and solid lipid nanoparticles for dermal application, Intl J Pharmaceutics 337(1–2) 291–298 (2007)
- V Jenning, A Gysler, M Schafer-Korting and SH Gohla, Vitamin A loaded solid lipid nanoparticles for topical use: Occlusive properties and drug targeting to the upper skin, European J Pharmaceutics and Biopharmaceutics 49(3) 211–218 (2000)
- JP Jee, SJ Lim, JS Park and CK Kim, Stabilization of all-trans retinol by loading lipophilic antioxidants in solid lipid nanoparticles, European J Pharmaceutics and Biopharmaceutics 63 134–139 (2006)
- SA Wissing and RH Müller, A novel sunscreen system based on tocopherol acetate incorporated into solid lipid nanoparticles, Intl J Cosmetic Science 23(4) 233–243 (2001)
- A Dingler, RP Blum, H Niehus, RH Müller and S Gohla, Solid lipid nanoparticles (SLN/ Lipopearls)—A pharmaceutical and cosmetic carrier for the application of vitamin E in dermal products, J Microencapsulation 16(6) 751–767 (1999)
- M Uner, SA Wissing, G Yener and RH Müller, Skin moisturizing effect and skin penetration of ascorbyl palmitate entrapped in solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) incorporated into hydrogel, Pharmazie 60(10) 751–755 (Oct 2005)
- M Uner, SA Wissing, G Yener and RH Müller, Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for application of ascorbyl palmitate, Pharmazie 60(8) 577–582 (2005)
- V Teeranachaideekul, RH Müller and VB Junyaprasert, Encapsulation of ascorbyl palmitate in nanostructured lipid carriers (NLC)—Effects of formulation parameters on physicochemical stability, Intl J Pharmaceutics 340(1–2) 198–206 (2007)
- E Cengiz, SA Wissing, RH Müller and Y Yazan, Sunblocking efficiency of various TiO2-loaded solid lipid nanoparticle formulations, Intl J Cosmetic Science 28(5) 371–378 (2006)
- R Tursilli, G Piel, L Delattre and S Scalia, Solid lipid microparticles containing the sunscreen agent octyl-dimethylaminobenzoate: Effect of the vehicle, European J Pharmaceutics and Biopharmaceutics 66(3) 483–487 (2007)
- J Wiechers and EB Souto, Formulating focus-delivering actives via solid lipid nanoparticles and nanostructured lipid carriers: Part II, nanoparticle characterization, Cosm & Toil 127 26–32 (Jan 2012)
- S Kuchler, M Abdel-Mottaleb, A Lamprecht, MR Radowski, R Haag, M Schafer-Korting, Influence of nanocarrier type and size on skin delivery of hydrophilic agents, Int J Pharm 30 377(1–2) 169–172 (Jul 2009)
- EB Souto, SA Wissing, CM Barbosa and RH Müller, Evaluation of the physical stability of SLN and NLC before and after incorporation into hydrogel formulations, European J Pharmaceutics and Biopharmaceutics 58(1) 83–90 (2004)
- R Yang et al, Preparation of gel-core-solid lipid nanoparticle: A novel way to improve the encapsulation of protein and peptide, Chemical & Pharmaceutical Bulletin 58(9) 1195–1202 (2010)
- A Lippacher, RH Müller and K Mader, Preparation of semisolid drug carriers for topical application based on solid lipid nanoparticles, Intl J Pharmaceutics 214(1–2) 9–12 (2001)
- A Lippacher, RH Müller and K Mader, Liquid and semisolid SLN dispersions for topical application: Rheological characterization, European J Pharmaceutics and Biopharmaceutics 58(3) 561–567 (2004)
- S Doktorovova and EB Souto, Nanostructured lipid carrier-based hydrogel formulations for drug delivery: A comprehensive review, Expert Opinion on Drug Delivery 6(2) 165–176 (2009)
- J Pardeike, A Hommoss and RH Müller, Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products, Intl J Phar- maceutics 366(1–2) 170–184 (2009)
- A Lippacher, RH Müller and K Mader, Investigation on the viscoelastic properties of lipid based colloidal drug carriers, Intl J Pharmaceutics 196(2) 227–230 (2000)
- M Krzic, M Sentjurc and J Kristl, Improved skin oxygenation after benzyl nicotinate application in different carriers as measured by EPR oximetry in vivo, J Controlled Release 70(1–2) 203–211 (2001)
- EB Souto, AJ Almeida and RH Müller, Lipid nanoparticles (SLN, NLC) for cutaneous drug delivery: Structure, protection and skin effects, J Biomedical Nanotechnology 3(4) 317–331 (2007)
- T Devringer and HAG Deronde, Preparation and structure of a water-in-oil cream containing lipid nanoparticles, J Pharmaceutical Sciences 84(4) 466–472 (1995)
- RH Müller, RD Petersen, A Hornmoss and J Pardeike, Nanostructured lipid carriers (NLC) in cosmetic dermal products, Advanced Drug Delivery Reviews 59 522–530 (2007)
- EB Souto, SA Wissing, CM Barbosa and RH Müller, Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery, Int J Pharm 18 278(1) 71–77 (Jun 2004)
- A Gulbake, A Jain, P Khare and SK Jain, Solid lipid nanoparticles bearing oxybenzone: In vitro and in vivo evaluation, J Microencapsulation 27(3) 226–233 (2010)
- SC Chi and HW Jun, Release rates of ketoprofen from poloxamer gels in a membraneless diffusion cell, J Pharmaceutical Sciences 80(3) 280–283 (1991)
- M Rafiee-Tehrani and A Mehramizi, In vitro release studies of piroxicam from oil-in-water creams and hydroalcoholic gel topical formulations, Drug Development and Industrial Pharmacy 26(4) 409–414 (2000)
- MR Bhalekar, V Pokharkar, A Madgulkar and N Patil, Preparation and evaluation of miconazole nitrate-loaded solid lipid nanoparticles for topical delivery, AAPS Pharmscitech 10(1) 289–296 (2009)
- W Mehnert and K Mader, Solid lipid nanoparticles— Production, characterization and applications, Advanced Drug Delivery Reviews 47(2–3) 165–196 (2001)
- M Bentinger, K Brismar and G Dallner, The antioxidant role of coenzyme Q, Mitochondrion 7 S41–S50 (2007)
- SF Martin, I Buron, JC Espinosa, J Castilla, JM Villalba and JM Torres, Coenzyme Q and protein/lipid oxidation in a BSE-infected transgenic mouse model, Free Radical Biology and Medicine 42(11) 1723–1729 (2007)
- Y Yoshida, M Hayakawa, Y Habuchi and E Niki, Evaluation of the dietary effects of coenzyme Q in vivo by the oxidative stress marker, hydroxyoctadecadienoic acid and its stereoisomer ratio, Biochimica Et Biophysica Acta-General Subjects 1760(10) 1558–1568 (2006)
- U Hoppe et al, Coenzyme Q(10), a cutaneous antioxidant and energizer, Biofactors 9(2–4) 371–378 (1999)
- M Jeya, HJ Moon, JL Lee, IW Kim and JK Lee, Current state of coenzyme Q(10) production and its applications, Applied Microbiology and Biotechnology 85(6) 1653–1663 (2010)
- Y Yue, HF Zhou, GL Liu, Y Li, ZM Yan and MX Duan, The advantages of a novel CoQ10 delivery system in skin photo-protection, Intl J Pharmaceutics 392(1–2) 57–63 (2010)
- Y Tanino et al, Decrease of antioxidants and the formation of oxidized diacylglycerol in mouse skin caused by UV irradiation, J Dermatological Science S21–S28 (2005)
- MW Genova et al, Mitochondrial production of oxygen radical species and the role of coenzyme Q as an antioxidant, Experimental Biology and Medicine 228(5) 506–513 (2003)
- JY Shin et al, Assembly of coenzyme Q10 nanostructure resembling nascent discoidal high density lipoprotein particle, Biochemical and Biophysical Research Communications 388(2) 217–221 (2009)
- B Siekmann and K Westesen, Preparation and physicochemical characterization of aqueous dispersions of coenzyme Q(10) nanoparticles, Pharmaceutical Research 12(2) 201–208 (1995) 5
- V Teeranachaideekul, EB Souto, VB Junyaprasert and RH Müller, Cetyl palmitate-based NLC for topical delivery of coenzyme Q(10)—Development, physicochemical characterization and in vitro release studies, European J Pharmaceutics and Biopharmaceutics 67(1) 141–148 (2007)
- EB Souto, SA Wissing, CM Barbosa and RH Müller, Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery, Intl J Pharmaceutics 278 (1) 71–77 (2004)
- JM Bourre, Dietary omega-3 fatty acids for women, Biomed Pharmacother 61(2-3) 105–112 (Feb-Apr 2007)
- G Chene et al, n-3 and n-6 polyunsaturated fatty acids induce the expression of COX-2 via PPARgamma activation in human keratinocyte HaCaT cells, Biochim Biophys Acta 1771(5) 576–589 (May 2007)
- HH Kim et al, Eicosapentaenoic acid inhibits UV-induced MMP-1 expression in human dermal fibroblasts, J Lipid Res 46(8) 1712–1720 (Aug 2005)
- RH Müller, A Hommoss, J Pardeike and C Schmidt, Lipid nanoparticles (NLC) as novel carrier for cosmetics—Special features and state of commercialization, SÖFW J 9 (2007)
- RH Müller, K Mader and S Gohla, Solid lipid nanoparticles (SLN) for controlled drug delivery— A review of the state of the art, European J Pharmaceutics and Biopharmaceutics 50(1) 161–177 (2000)
- DG Kim et al, Retinol-encapsulated low molecular water-soluble chitosan nanoparticles, Int J Pharm 319 130–138 (2006)
- J Varani et al, Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin, J Invest Dermatol 114 480–486 (2000)
- M Nighland, M Yusuf, S Wisniewski, K Huddleston and J Nyirady, The effect of simulated solar UV irradiation on tretinoin in tretinoin gel microsphere 0.1% and tretinoin gel 0.025%, Cutis 77 313–316 (2006)
- KA Shah, AA Date, MD Joshi and VB Patravale, Solid lipid nanoparticles (SLN) of tretinoin: Potential in topical delivery, Int J Pharm 345 163–171 (2007)
- PV Pople, KK Singh, Development and evaluation of topical formulation containing solid lipid nanoparticles of vitamin A, AAPS PharmSciTech 7 91 (2006)
- ME Carlotti, E Ugazio, S Sapino, E Peira, M Gallarate, Photodegradation of retinol and anti-aging effectiveness of two commercial emulsions, J Cosmet Sci 57 261–277 (2006)
- GA Castro, CA Oliveira, GA Mahecha and LA Ferreira, Comedolytic effect and reduced skin irritation of a new formulation of all-trans retinoic acid-loaded solid lipid nanoparticles for topical treatment of acne, Arch Dermatol Res 303 513–520 (2011)