Traditionally it has been a standard practice to use numerical nomenclature within stratum corneum (SC) ceramide literature. This nomenclature is based on the relative polarity of ceramides separated by high performance thin-layer chromatography (HPTLC) Rf values to classify the ceramide species. However, this practice is fundamentally flawed and is continually undermined as improved separation techniques are revealing ceramides that co-migrate by HPTLC. As previously unrecognized ceramide species are discovered, it has become necessary to rename all the ceramide species according to their new relative Rf values. As it turns out, this numbering system then becomes complicated because it is no longer based on ceramide polarity alone.
The confusion regarding ceramide nomenclature can be eliminated by adopting a nomenclature based on the actual chemical structure of ceramides instead of their chromatographic location. This idea was first suggested some years ago1, 2 and is now widely practiced in the skin barrier literature. SC ceramide chemical nomenclature is based on four molecular structures corresponding to the base chains: sphingosine (S), dihydrosphingosine (DS), 6-hydroxy sphingosine (H), and phytosphingosine (P). The presence of a saturated fatty acid is represented by “N,” an alpha hydroxy group on the acid chain is represented by “A,” an omega hydroxy group is represented by “O,” and an ester linkage by “E”. The complete molecule is named “ceramide-acid-base,” i.e., the acid chain abbreviation precedes the base chain. In the case of an ester link in the acid chain, this is noted first. For example, a 6-hydroxy sphingosine with an alpha hydroxy fatty acid chain would be denoted as “Cer [AH].” A comparison of the old numbering system with the new nomenclature system is given in Figure 2.
Article 6 of the Cosmetics Directive3 lists labeling requirements for cosmetic products, namely the International Nomenclature Cosmetic Ingredient (INCI) names. This is important because INCI declarations are used by consumers to make product purchase judgements since efficacy is associated with given ingredients. Recently, the new INCI nomenclature for the INCI labeling of ceramides was agreed upon by the Personal Care Products Council so that it is now consistent with the scientific literature. The newer ratified nomenclature is based on work published by Motta et al.4 and will primarily be used in the discussion that follows.
Background, Isolation and Elucidation of Ceramide Structure
First, what is a ceramide? Ceramides are amide-linked fatty acids containing a sphingoid base, which can be described as long-chain amino alcohol derived from the condensation of the amino acid L-serine and palmitoyl-CoA. Normal, alpha hydroxyl, omega hydroxyl and unsaturated fatty acids are found in ceramides. The amino base is called a sphingoid base and can consist of phytosphingosine, sphingosine, dihydrosphingosine and 6-hydroxysphingosine. These typical structures are shown in Figure 1. From this structure it can be deduced that the sphingoid base has up to four asymmetric carbon atoms, suggesting the possibility of up to sixteen isomer formations depending on the sphingoid base. However, the sphingoid base structure in nature is restricted to the 2S, 3R-(or erythro) conformation, which is derived from the L-serine part; for instance (2S, 3R, 4E)-2-)N-acylamino)-4-octadecene 1, 3 diol or N-acyl-D-erythro-sphingosine.
Naturally, as one would expect, this conformation is key for interacting with other naturally occurring lipids, especially those in the SC. Although SC ceramides are highly complex—see Figure 2 showing the main intercellular and solvent-extractable ceramide species—how and why were they identified? Early researchers recognized that the epidermis was more impermeable than the dermis but it was not until the early 1940s that the precise location of the barrier was thought to reside in the SC. Twenty years later using organic solvent extraction of the SC, Matoltsy et al.5 demonstrated that lipids played a critical role in barrier function.
Then in 1965, Nicolaides6 identified ceramides as a quantitatively significant polar SC lipid. Nevertheless, this finding was largely unappreciated until the mid 1970s when Yardley and Gray published the complete lipid composition of human SC; their detailed and pioneering work7,8 in the mid to late 1970s was pivotal and catalytic to the whole field of skin ceramide research. Until this point, most skin lipid research had focused on phospholipids, which could not be involved in the water permeability barrier since they are almost absent from the SC.
While Gray and Yardley9 were identifying a number of glycolipids present in skin and discovering a unique epidermal glycolipid rich in linoleic acid, Elias and Friend10 were visualizing the bilayer nature of lipids between SC cells by using electron microscopic techniques. At that point it was becoming apparent that the SC intercellular ceramides represented a structurally heterogeneous class of lipids containing normal and hydroxyl acids, together with sphingosine and phytosphingosine as the major components. Subsequently, their detailed structures were elucidated by Downing’s team, and especially by Phil Wertz in the 1980s.11, 12
Building on Gray’s biochemical studies, two main research centers then elucidated the structures of the ceramides and their precursors present in the epidermis. Bowser and co-workers13 determined unequivocally the structure of the unique epidermal glycolipid acylglucosylceramide but proved that in itself it could not be a barrier lipid because it was absent from the stratum compactum, which is the tightly compact lower layers of the SC. Simultaneously with the Downing and Wertz group14, Bowser et al. 13 discovered a new class of structurally related ceramides, one of which was rich in linoleic acid and present in the stratum compactum—Ceramide 1 or acylceramide (see Figure 2). Comparing the structures of the glucosylceramides indicated that they were precursors of related ceramides with acylglucosylceramide being the precursor of Ceramide 1. Much later, metabolic studies supported this as fact.15
Following this work, several other types of glucosylceramides and ceramides also were discovered. In addition to the major linoleoyl-rich species, five chromatographically distinct ceramide and glucosylceramide classes could be separated and isolated by thin-layer chromatography. For the SC ceramides, these were named according to their polarity, beginning with Ceramide 1 for the most nonpolar and linoleic acid-containing species (see Figure 3).
These lipids consist of C16-C22 sphingosine (trans-4-sphingenine), dihydrosphingosine and phytosphingosine (4-D-hydroxy sphingosine) bases amidated to long chain fatty acids, which may or may not be α- or Ω-hydroxylated and unsaturated. As mentioned, the Ω-hydroxyl of Ceramide 1 was further shown to be esterified to fatty acids, with linoleic acid as the major acid present. This also occured for porcine Ceramide 6a; however, the corresponding species in human SC consisted of a phytosphingosine ceramide in which the base hydroxyl was further esterified to a hydroxy fatty acid.
Robson et al.16 and Vietzke et al.17 demonstrated that seven of the thin layer, chromatographically migrating ceramide bands corresponded to nine ceramide classes because two bands contained two co-migrating ceramide species, including one at the
Ceramide 2 position, which was N-acyldihydrosphingosine. Robson et al.14 also identified an Ω-hydroxy acid derivative of a new base, 6-hydroxy-4-sphingenine. Vietzke et al.17 demonstrated that two ceramides co-migrated in the Ceramide 4 position. Equally Vietze et al. speculated on the presence of an Ω-hydroxy fatty acid-containing phytosphingosine derivative but this tenth ceramide species was identified by Ponec et al.18 in 2003. The fatty acid chain length variation of acylceramides has been the subject of research by Farwanah et al., where longer chain variants with odd fatty-acid chain lengths have been described.19
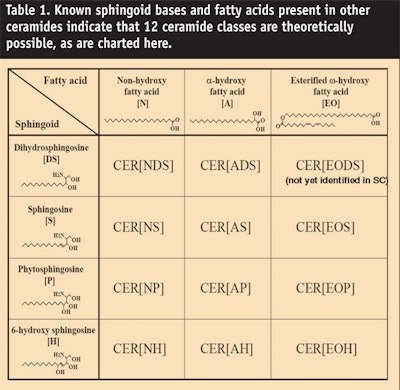
Most recently Masukawa et al.20 identified an 11th ceramide species consisting of an α-hydroxyl acid amidated to dihydrosphingosine (see Figure 3). In addition, using normal phase liquid chromatography connected to electrospray ionization mass spectrometry, the same group identified 342 different species within these 11 ceramide classes. Nevertheless, Table 1 and Figure 4 indicate that 12 species are theoretically possible based upon known sphingoid bases and fatty acids present in other ceramides. So far, the 12th ceramide remains elusive. For now, there are only 11 players but they demonstrate tremendous heterogeneity.
Epidermal Biosynthesis of Ceramides
Ceramide synthesis occurs during epidermal differentiation, during which all SC lipids are packaged into lamellar granules (see Figure 5). However, serine palmitoyl transferase is the rate-limiting enzyme in sphingolipid synthesis (see Figure 6).
In a nicotinamide adenine dinucleotide phosphate (NADPH)-dependent reaction, serine palmitoyl transferase condenses palmitoyl CoA to produce 3-ketodihydrosphingosine, which is then reduced to dihydrosphingosine by an NADPH reductase. Subsequently N-acylation produces a dihydroceramide. These ceramides can then be hydroxylated to produce phytosphingosine-containing ceramides, or a trans 4-5 double bond can be introduced to produce sphingosine-containing ceramides. Following, sphingosine-containing ceramides can be hydroxylated to produce 6-hydroxysphingosine derivatives. Ceramides in this state cannot be transported easily into living cells because they occur as crystalline lipids with very high melting points. Therefore they are glucosylated in the Golgi apparatus by glucosylceramide synthase or are converted to sphingomyelins by sphingomyelin synthase and packaged into lamellar granules. During differentiation these granules are extruded into the SC intercorneocyte spaces and sphingolipids are acted upon by sphingomylinases and β-glucosylcerebrosidase to produce the mature ceramides in the SC. In the de-glycosylated state these ceramides, together with other SC lipids, form the typical orthorhombically packed lamellar lipids known to occur in the SC.1 Taken together it is obvious that keratinocytes spend significant metabolic resources and energy for the production and transport of ceramides for the creation of a properly functioning skin barrier.
Electron microscopy studies have facilitated tremendous understanding of the repeating lamellar patterns of the SC intercellular lipids. However, the repeat lamellar states and especially the lateral packing states can be best observed by X-ray diffraction studies. Lipids in vivo appear to exist as a balance between a solid crystalline state (orthorhombic packing) and gel (hexagonal packing) or liquid crystalline states. The former represents the most tightly packed conformation with optimal barrier properties but a greater proportion of hexagonally packed lipid conformations are known to occur in the outer layers of the SC, presumably to facilitate desquamation.
Ceramides and cholesterol alone are capable of forming the hexagonal phase but the formation of the orthorhombic phase also requires the presence of long chain fatty acids. Repeating lamellar lipid phases also have been observed—the long periodicity phase (LPP) and short periodicity phase (SPP), as well as a unique liquid phase. From this work, Bouwstra proposed the sandwich model;21 however, one particular Ω-6-containing linoleate-enriched ceramide (CER EOS, see Figure 2) has been shown as crucial in forming the LPP, the liquid phase and the orthorhombic phase that is essential for barrier function. As a consequence, greater amounts of these phases are observed mainly with linoleate-containing CER EOS, less with oleate-containing CER EOS and are totally absent if only stearate-containing CER EOS is present in the lipid mixtures.
Problem Skin and Its Treatment
It is now well-established that, in hyperproliferative disorders such as aged dry skin and even in dry skin of the aged, an increase in epidermopoiesis is found that is relative to the younger control group, and there is a change in SC lipid levels and composition.22 Several groups have published on the reductions of ceramides in dry and aged skin. Changes in the composition of the ceramide sub-types are reported to occur and a predominance of sphingosine-containing ceramides at the expense of the phytosphingosine-containing ceramides has been observed in the SC of subjects with dry skin.23
Most recently shortening and lengthening of the acyl sphingoid bases sphingosine and 6-hydroxysphingosine was reported in dry skin.24 These changes in lipid composition will of course influence the lamellar packing of the lipids and a reduction of CER EOS and EOH with increased concentrations of sphingosine-containing ceramides (CER NS and CER AS) and crystalline cholesterol, in association with a loss of the LPP in dry skin. However, although the lipid ultrastructure is clearly aberrant in the outer layers of dry skin, more work is needed to ascribe a particular lipid phase.
Overall, SC hydration needs to be improved in dry, aged skin. Equally, the lipid lamellar architecture in the outer layers of the SC needs to be normalized in dry, flaky skin conditions and the compositional changes including lowered levels of ceramides, especially the phytosphingosine-containing ceramides and CER EOS, need to be corrected. Evidence also indicates that a reduction in long chain fatty acids occurs in SLS-induced dry skin. Since these lipids are important for inducing an orthorhombic lateral packing state, these will also need to be supplied to the skin to more effectively correct barrier function.
Several clinical studies evaluating the effects of ceramide technologies recently were conducted. It is important to note that the full benefits of a ceramide technology formulated into heavy emulsions where other emollients dominate the formulation are difficult to discern unless the ceramide technologies are used at a high enough concentration.
Nevertheless, the properties of Locobase Repair cream, a commercial product tested by Berardesca et al.,25 were investigated in two studies that found opposite effects on barrier recovery. Barany et al.26 did not find improvements to a placebo, whereas Kucharekova et al.27 found that the cream containing CER NP significantly reduced TEWL, erythema and epidermal proliferation compared with a placebo cream. Further improvements in function have been observed with complete lipid mixtures. De Paepe et al.2 demonstrated improvements in barrier functionality and SC hydration from a lipid mixture of: 0.2% CER NP, 0.1% CER AS and 0.2% CER NP (unsaturated), together with 0.25% cholesterol, 0.25% linoleic acid and 0.5% phytosphingosine; when compared with placebo lotions and a lotion containing only 0.6% CER NP and 0.4% CER NP (unsaturated).
Berardesca et al.25 also have established that balanced lipid mixtures containing CER NP are effective in improving the barrier properties and clinical condition of skin in subjects with contact dermatitis. Equally, Chamlin et al.28 showed that a ceramide-dominant barrier repair lipid cream alleviates childhood atopic dermatitis. Over a six-week treatment period, TEWL values were found to decrease by 50% and the number of D-squame tape-strippings required to break the barrier increased from approximately 12 to 22 strippings, indicating a stronger SC barrier function. Wollenweber et al.29 also studied the effects of applying a lipid concentratea designed for enhanced skin moisturization and protection, and found that barrier repair and long-term hydration were increased.
Equally important, however, was the correct stereochemistry of the ceramides to form the SPP and LPP phases that are normally found in the SC. Chiral CER NP form the correct SPP and LPP phases, together with other skin lipids, while a racemic CER NS disrupted the lipid matrix, resulting in a completely different X-ray diffraction profile and, in particular, the loss of the LPP. Equally, Farwick et al. recently demonstrated that a mixture of CER EOS, EOP, NS, NP and AP, together with nonanimal-derived cholesterol and behenic acid, can also mimic the lamellar ordering of human SC lipids known to be the most favorable conformation for skin protection.30
Nevertheless, it remains important to note that the stereochemistry of ceramides needs to be considered. Wollenweber et al. demonstrated29 that racemic ceramides cannot form the correct SC lamellar lipid structure found in the SC, which was further exemplified by Farwick et al. for mixtures of ceramides.30
Conclusion
Ceramides have been and will continue to be important ingredients for the cosmetic industry. Leaps have been made in understanding their structure, function, biosynthesis and chemical synthesis over the last two decades, especially with regard to their levels, composition and packing structures in dry and aged skin, together with the need for skin-identical ceramides with the correct stereochemistry to mimic the correct lamellar structure found in the SC.
However, not all ceramides have the same structure and thus there has been a need to clarify the nomenclature in the scientific literature, as well as on cosmetic product labels. A new INCI labeling system was recently approved by the Personal Care Products Council and based on the system of Motta et al. The new SC ceramide chemical nomenclature is based on four molecular structures corresponding to the base chains; sphingosine (S), dihydrosphingosine (DS), 6-hydroxy sphingosine (H), and phytosphingosine (P). The presence of a saturated fatty acid is represented by “N,” an alpha hydroxy group on the acid chain is represented by “A”, an omega hydroxy group is represented by “O” and an ester linkage by “E.” The complete molecule is named according to: ceramide-acid-base; i.e., the acid chain abbreviation precedes the base chain (in the case of an ester link in the acid chain, this is noted first).
Certainly more ceramides will be identified in the future but these will be most likely derived from the four main sphingoid base structures described in the present article. This nomenclature system will help to avoid confusion of the types of ceramides that are used by formulators and will help them to make better judgements of which types of ceramides to use in products for different skin treatments.
References.
1. WM Holleran and Y Takagi, Stratum corneum lipid processing: The final steps in barrier formation, in Skin Barrier, PM Elias and KR Feingold, eds, New York: Taylor and Francis (2006) Ch 15, pp 231–260
2. K De Paepe, D Roseeuw and V Rogiers, Repair of acetone- and sodium lauryl sulphate-damaged human skin barrier function using topically applied emulsions containing barrier lipids, JEADV 16, 587–594 (2002)
3. European Union Web site, available at: eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:1976L0768:20080424:EN:PDF (Accessed Nov 24, 2008)
4. S Motta, M Monti, S Sesana, R Caputo,
S Carelli and R Ghidoni, Ceramide composition of the psoriatic scale, Biochim Biophys Acta1182(2) 147–51 (Sep 8, 1993)
5. AG Maltosky, H Schragger and MN Matolsky, Observations on regeneration of the skin barrier, J Invest Dermatol 38 251 (1962)
6. N Nicolaides, Skin lipids II: Lipid class composition of samples from various species and anatomical sites, J Am Oil Chem Soc 42 691–702 (1965)
7. GM Gray and HJ Yardley, Different populations of pig epidermal cells: Isolation and lipid composition, J Lipid Res 16 441–447 (1975)
8. GM Gray and HJ Yardley, Lipid composition of cells isolated from pig, human and rat epidermis, J Lipid Res 16 434–440 (1975)
9. GM Gray and HJ Yardley, Glycosphingolipids and ceramides in human and pig epidermis,
J Invest Dermatol 70 336–341 (1978)
10. PM Elias and DS Friend, The permeability barrier in mammalian epidermis, J Cell Biol 65 180–191 (1975)
11. PW Wertz and DT Downing, Ceramides of pig epidermis: Structure determination, J Lipid Res 24 759–765 (1983)
12. PW Wertz et al, Composition of the ceramides from human stratum corneum and from comedones, J Invest Dermatol 84 410–412 (1985)
13. PA Bowser, DH Nugteren, RH White et al, Identification and characterization of epidermal lipids containing linoleic acid, Biochim Biophys Acta 834 (3) 419–28 (1985)
14. PW Wertz and Downing DT, Acylglucosylceramides of pig epidermis: Structure determination, J Lipid Res 24(6) 753–6 (1983)
15. KC Madison, DC Swartzendruber, PW Wertz and DT Downing, Murine keratinocyte cultures grown at the air/medium interface synthesize stratumcorneum lipids and “recycle” linoleate during differentiation, J Invest Dermatol 93(1) 10-7 (Jul 1989)
16. KJ Robson et al, 6-hydroxy-4-sphingenine in human epidermal ceramides, J Lipid Res 35 2060–2068 (1994)
17. JP Vietzke et al, Comparative investigation of human ceramides, Lipids 36(3) 299–304 (2001)
18. M Ponec et al, New acylceramide in native and reconstructed epidermis, J Invest Dermatol 120 581–588 (2003)
19. H Farwanah et al, E-publication (Feb 24, 2007), Separation and mass spectrometric characterization of covalently bound skin ceramides using LC/APCI-MS and Nano-ESI-MS/MS, J Chromatogr B Analyt Technol Biomed Life Sci 852(1-2) 562–70 (Jun 1, 2007).
20. Y Masukawa, H Narita, E Shimizu et al, Characterization of overall ceramide species in human stratum corneum, J Lipid Res 49(7) 1466–76 (Jul 2008)
21. JA Bouwstra, FER Dubbelaar, GS Gooris and M Ponec, The lipid organization in the skin barrier, Acta Derm Venereol Suppl 208 23–30 (2000)
22. AV Rawlings, Trends in stratum corneum research and the management of dry skin conditions, Int J Cosmet Sci 25 (1-2) 63–95 (2003)
23. AW Fulmer and GJ Kramer, Stratum corneum lipid abnormalities in surfactant-induced dry scaly skin, J Invest Dermatol 86(5) 598–602 (May 1986)
24. M Chopartet al, Quantitative analysis of ceramides in stratum corneum of normal and dry skin, Stratum Corneum III (2001)
25. E Berardesca, M Barbareschi, S Veraldi and N Pimpinelli, Evaluation of efficacy of a skin lipid mixture in patients with irritant contact dermatitis, allergic contact dermatitis or atopic dermatitis: A multicenter study, Contact Derm 45, 280–5 (2001)
26. E Barany, M Lindberg and M Loden, Unexpected skin barrier influence from nonionic emulsifiers, Int J Pharm 195, 189–195 (2000)
27. M Kucharekova, J Schalkwijk, PC Van De Kerkhof and PG Van De Valk, Effect of a lipid-rich emollient, Contact Derma 46, 331–338 (2002)
28. SL Chamlin et al, Ceramide-dominant barrier repair lipids alleviate childhood atopic dermatitis: Changes in barrier function provide a sensitive indicator of disease activity, J Am Acad Dermatol 47, 198–208 (2002)`
29. U Wollenweber, K Korevaar, AV Rawlings and Schick, Application of a skin-identical lipid concentrate for enhanced skin moisturization and protection, SÖFW 130 (Sep 2004)
30. M Farwick et al, An aquaporin-inspired lipid concentrate for mature skin, Cosm & Toil 123 (4)69–74 (2008)