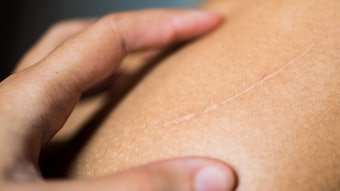
Many consumers have scars, sunspots or other unwanted dark spots that seem to have appeared overnight. These are all caused by hyperpigmentation, i.e., an increase in melanin production as a direct result of damage. Whether it is an old scar on the knee, or freckles scattered across the shoulders from an afternoon at the beach, both are caused by alterations in melanogenesis.
Melanin is the inherent compound responsible for pigmentation and it is found in the hair, skin and eyes. It plays an important role in protecting skin from the harmful effects of UV radiation and in scavenging toxic drugs and chemicals. Biological agents interfere with pigmentation through different mechanisms—ranging from interaction during the initial stages of melanogenesis, to the destruction of the melanocyte—and this article will focus on the key ones: tyrosinase inhibition, maturation and degradation; MITF inhibition; downregulation of MC1R; interference with melanosome transfer; and desquamation and peeling.
Tyrosinase Expression
In order to understand the mechanisms behind hypopigmenting agents, it is essential to comprehend the process of pigmentation. Melanogenesis is the natural process through which the human body creates melanin. This process is enhanced by the activation of key enzymes in response to environmental stress. Tyrosinase, the most-studied of the enzymes involved in melanogenesis, is a glycoprotein located in the membrane of the melanosome, a minifactorial vesicle within the melanocyte.1 Tyrosinase catalyses the first two steps of melanin production: the hydroxylation of L-tyrosine to L-dihydroxyphenylalanine (L-DOPA), and the subsequent oxidation of this o-diphenol to the corresponding quinone, L-dopaquinone. Even though L-tyrosine is the building block of melanin, it can only be transported into the melanosome by facilitated diffusion.2–5
Once dopaquinone is formed, the melanin pathway divides between the synthesis of pheomelanin and eumelanin.6 Dopaquinone converted through auto-oxidation leads to dihydroxyindole, or dihydroxyindole-2-carboxylic acid (DHICA), to form eumelanin, the dark-brown pigment. Or, dopaquinone is converted to cysteinyl DOPA or glutathione DOPA in the presence of cysteine or glutathione, which leads to the production of pheomelanin, a yellow-red pigment.
Melanocytes located in the basal lamina synthesize organelles called melanosomes, which contain melanin. Following synthesis, the melanosome is transferred to the keratinocytes. Keratinocytes that contain melanin granules then proceed along the differentiation pathway, moving up through the epidermal layers until they become part of the stratum corneum (SC).
Microphthalmia-associated transcription factor: Considering the described pathway, it is clear that a decrease in the intracellular levels of tyrosinase and other melanogenic enzymes would reduce the amount of melanin produced during pigmentation. An approach targeting this mechanism is via Microphthalmiaassociated transcription factor (MITF). MITF belongs to the basic helix-loophelix- zip family of transcription factors that regulates melanocyte proliferation and melanogenesis. MITF is the major regulator of tyrosinase and related enzymes (TRPs) as well as various melanosomal structural proteins such as pMel17.7
Transforming growth factor (TGF- β1) is a polypeptide growth factor that has been shown to inhibit melanin production by downregulating MITF.8, 9 TGF-β1 is also capable of interfering with the synthesis of tyrosinase and with the intracellular stability of the protein itself.8 However, a similar effect can be achieved by application of C2 Ceramides, as they are also capable of activating MTIF degradation or blocking MITF expression mediated by an initial effect on the AKT/PKB cell signaling pathway.9
Essential fatty acids: Essential fatty acids are another class of naturally occurring compounds capable of providing hypopigmenting effects. However, their effects can be considered complex and at some level, confusing given that very different results can be obtained from the saturated forms just as the unsaturated forms.10 For example, the unsaturated linoleic acid decreases tyrosinase activity, while the saturated palmitic and stearic acids increase it.10 A study was conducted to measure the effects of some fatty acids in guinea pig skin following stimulation with UV light. Linolenic, linoleic and oleic acids showed a bleaching effect; however, neither the number of melanosomes nor the level of tyrosinase mRNA were influenced. Therefore, the inhibition of melanin was most likely caused by a decrease in the amount of active tyrosinase contained in the melanocytes, caused by the stimulation of tyrosinase ubiquitination and degradation by the proteasome.11
α-Tocopherols: α-Tocopherols also have been tested as hypopigmenting agents; however, their effects are not attributed to the direct inhibition of tyrosinase activity but instead to an apparent decrease in hyperpigmentation.12 Vitamin E, a well-known antioxidant, is capable of increasing the synthesis of glutathione, the major endogenous antioxidant produced by cells.13 Glutathione is capable of derivating dopaquinone to pheomelanin, thus as more dopaquinone is branched to pheomelanin synthesis, less eumelanin is accumulated, leading to a decrease in apparent skin pigmentation due to the lighter color of pheomelanin.13
Tocopherols conjugated with phenols at position 4, for example resorcinol, are capable of providing a double hypopigmenting effect. They can inhibit tyrosinase by their phenol moiety while reducing apparent pigmentation and providing the ROS scavenging properties of the α-tocopherol moiety.14
Controlling Melanocortin Receptor 1
The second approach to reducing hyperpigmentation is through the interaction with the melanocortin receptor 1 (MC1R). MC1R, a melanocyte surface receptor, may be an effective way to alter pigment production. This is due to the fact that the alpha-melanocyte stimulating hormone (α-MSH) has been shown to bind to the MC1R and induce eumelanin production. In contrast, the agouti signal protein (ASP) is capable of binding to the MC1R to prevent the formation of eumelanin by inhibiting α-MSH and in turn stimulating the production of pheomelanin. Therefore, the reduction in hyperpigmentation is achieved by a direct competition at the binding site and a down-regulation of the receptor signaling. By using peptides that are capable of binding to the MC1R, much like α-MSH and ASP, it is possible to alter melanin formation and enhance the production of one type of melanin while inhibiting the production of the other.
A Lactobacillus-derived peptide, for example, has been developed to prevent eumelanin synthesis while simultaneously increasing pheomelanin synthesis to improve skin tone and reduce the appearance of hyperpigmentationa. The efficacy of this peptide was first assessed in vitro using an assayb to measure the amount of melanin content per tissue as well as per tissue weight. Compared to kojic acid, the ingredient was capable of stimulating lower concentrations of visible melanin per tissue. An MTT assay was then performed to determine any potential immunotoxic effects; results indicated the ingredient to be safe for use in cosmetics and personal care products.15, 16
Melanosome Manipulation
As previously discussed, once melanin is synthesized in cells, it is stored in melanosomes that are then transferred to keratinocytes. Keratinocytes that contain melanin then move up through the epidermal layers until they become part of the SC, where the pigment becomes visible. For this reason, interfering with melanosome maturation or transfer is another means to reduce the appearance of hyperpigmentation. A novel mechanism for the regulation of pigmentation is through the inhibition of the keratinocyte receptor proteaseactivated receptor 2 (PAR-2). The inhibition of serine proteases results in an impaired activation of this receptor on the keratinocyte, which leads to the accumulation of melanosomes within the melanocyte.17 Therefore, the inhibition of PAR-2 can block the transfer between cells and in turn block the dispersion of pigment to the keratinocytes.
An example of an ingredient that can provide this effect is yarrow extract. Achillea millefolium or yarrow is a flowering plant native to the Northern Hemisphere. This perennial plant is rich in centaureidine, a flavonoid glucoside that reduces dendrite growth and the transfer of melanosomes to keratinocytes. Movement of melanosomes along melanocyte dendrites is necessary for the transfer of melanin pigment from melanocytes to basal and suprabasal keratinocytes. Therefore, a reduction in the growth of melanocyte dendrites reduces the amount of melanin that is transferred to the outer layers of the skin in the keratinocytes.18
Plasma membrane lectins and their glycol-conjugates are also critical molecules involved in this transfer process. Minwalla et al.19 investigated the effects of these molecules on the viability of melanocytes and keratinocytes and on the reversibility of melanosome-transfer inhibition induced by these agents using an in vitro melanocyte-keratinocyte coculture model system. Results indicated that lectins and neoglycoproteins were capable of inducing apoptosis in a dosedependent manner to melanocytes or keratinocytes in monoculture. The dosages at which lectins did not affect cell viability produced an inhibitory effect on melanosome transfer when used alone or in co-cultures of melanocyteskeratinocytes. Co-cultures treated with lectins resumed normal melanosome transfer in three days after the removal of the inhibitor, proving the reversibility of this effect.19
Depigmenting Agents
Since the original reports of Olivier, Schwartz and Warren in the early 1940s describing the depigmenting effects of monobenzyl ether of hydroquinone, a notable number of phenolic compounds have been evaluated as inhibitors of melanin synthesis.20 For this reason, herbal extracts of botanicals rich in phenols, flavonoids, coumarins and other derivatives have gained significant attention as potential hypopigmenting agents.
Hydroquinone is a well-known tyrosinase inhibitor. Its effectiveness was discovered when it was observed that men of color who worked in the tanning industry presented occupational leukoderma, an acquired condition with localized loss of pigmentation in the skin.20 This condition was associated with the use of rubber gloves in the work area. Back then, the rubber industry added an antioxidant of pigment called monobenzyl ether of hydroquinone to rubber gloves to prevent their aging, which was causing leukoderma.20
Hydroquinone’s effectiveness to reduce hyperpigmentation is attributed to its ability to inhibit tyrosinase through interaction with copper at the active site and alteration of melanosomal functions. However, numerous studies showed the ingredient’s cytotoxicity at high usage levels, i.e., > 5.0%, as well as adverse effects to the skin, such as permanent hypomelanosis.21, 22 For these reasons, this ingredient is now banned from use in cosmetic products in the European Union and is allowed only in prescribed products in the United States. As a result, scientists continue the search for alternatives to provide bleaching effects through the use of chemical agents.
Arbutin, a natural β-glycoside of hydroquinone, was the first alternative to hydroquinone discovered (see Figure 1). As a derivative of hydroquinone, it is a good inhibitor of tyrosinase, although it does not affect its expression and synthesis in culture.23 Arbutin does, however, provide a much milder lightening effect than hydroquinine due to its controlled release by the in vivo hydrolysis of the glucosidic bond. α-Glucosides of arbutin have been chemically synthesized to provide stronger de-pigmenting activity, which is credited to their higher availability as well as ability to release hydroquinone.24 Deoxyarbutin is another derivative of arbutin that was developed to augment its effectiveness. It is synthesized by removing the hydroxyl groups from arbutin.25 Deoxyarbutin has been identified as an excellent tyrosinase inhibitor; in fact, in a study on guinea pig skin, it demonstrated rapid and sustained skin lightening that was completely reversible within eight weeks of application. This reversibility of the lightening effect indicates the melanocyte is not permanently damaged, but also the need for continued application to achieve sustained results.
The bleaching effects of deoxyarbutin were proven to be more sustained than those of hydroquinone and arbutin. In a clinical trial, the application of deoxyarbutin for 12 weeks showed a significant reduction in the lightness and improvement of solar lentigines, illustrating its potential to inhibit tyrosinase and provide skinlightening benefits as well as effectively reduce hyperpigmentary lesions.25 This effect can be explained by its unique chemical structure. The modulation of melanogenesis in the melanocytes can be achieved using chemicals that share structural homologies with tyrosine and as such, competitively inhibit the catalytic function of tyrosinase. Deoxysugars are a class of compounds that significantly increase the skin penetration and binding affinity for tyrosinase and thus inhibit its activity.25
Besides tyrosinase activity, expression and stability, alternative approaches use antioxidants to inhibit the cascade of chemical reactions leading to pigment formation. This process is through a modification in the type of melanin formed or interference with the melanosome transfer and pigment distribution. Lipophilic compounds with antioxidant capabilities such as lipoic acid are being used as depigmenting agents due to their involvement in the polymerization of melanogenic intermediates to produce melanin.26, 27 Lipoic acid, as well as its reduced form, dihydrolipoic acid, is capable of blocking MITF expression and inhibits the activation of the NF-kB transcription factor. Therefore, its bleaching effect is attributed to dopaquinone trapping and tyrosinase inhibition.28
Finally, desquamating agents are used for skin lightening. The continuously growing demand for skin lighteners is associated with preexisting darkening. Thus, hypopigmenting agents need not only prevent the formation of new melanin by blocking melanogenesis, but also reduce the existing pigmentation. Ingredients that increase skin desquamation are ideal for this application as this process accelerates the loss of melanin by peeling off the SC cells.
Retinoids such as retinoic acid are excellent desquamating agents and their hypopigmenting benefits are not only attributed to this property, but also to their ability to induce the dispersion of keratinocyte pigment granules and interfere with pigment transfer.29 However, their lightening effects can be considered somewhat slow compared to those of other depigmenting chemical agents such as glycolic, salicylic and lactic acids. These organic acids have been shown to effectively reduce the appearance of areas of hyperpigmentation such as freckles, actinic lentigines, melasma and post-inflammatory hypermelanosis. This effect is achieved by the peeling of the corneocytes and the epidermal upper layer keratinocytes.30
A 12-week study on subjects with medium to dark skin indicated that lactic acid supplemented with 1% ascorbic acid provided a stronger whitening effect that is perceived after three months of continuous application.31 Another study indicated that salicylic acid is effective in inducing skin desquamation, especially in dark-skinned individuals by a noncompetitive inhibition of tyrosinase.32
Conclusions
As has been discussed throughout the article, hypopigmenting agents provide whitening effects by interfering with the different stages of melanogenesis. For this reason, effective treatments combine two or more agents acting on different mechanisms, creating a synergistic hypopigmenting effect. Nevertheless, there are other factors that should be considered to ensure the development of a successful product. These factors include the stability of the individual ingredients as well as the way they interact with the other ingredients in the formula; their toxicity and skin penetration; global regulations; and the definition markers and targets for evaluating the effects of the ingredients to ensure sustained effectiveness and competition in the market.
References
- BS Kwon, AK Haq, SH Pomerantz and R Halaban, Isolation and sequence of a cDNA clone for human tyrosinase that maps at the mouse c-albino locus, Proc Natl Acad Sci USA 84(21) 7473–7477 (1987)
- G Prota, The role of peroxidase in melanogenesis revisited, Pigment Cell Res Suppl 2, 25–31 (1992)
- VJ Hearing, Unraveling the melanocyte, Am J Hum Genet 52(1) 1–7 (1993)
- S Ito, K Fujita, H Takahashi and K Jimbow, Characterization of melanogenesis in mouse and guinea pig hair by chemical analysis of melanins and of free and bound dopa and 5-S-cysteinyldopa, J Invest Dermatol 83(1) 12–14 (1984)
- KG Strothkamp, RL Jolley and HS Mason, Quaternary structure of mushroom tyrosinase, Biochem Biophys Res Commun 70(2) 519–524 (1976)
- K Jimbow, F Alena, W Dixon and H Hara, Regulatory factors of pheo- and eumelanogenesis in melanogenic compartments, Pigment Cell Res Suppl 2, 36–42 (1992)
- E Steingrimsson, NG Copeland and NA Jenkins, Melanocytes and microphthalmia transcription factor network, Annu Rev Genet 38 365–411 (2004)
- M Martinez-Esparza, C Jimenez-Cervantes, F Solano, JA Lozano and JC Garcia-Borron, Mechanisms of melanogenesis inhibition by tumor necrosis factor-alpha in B16/F10 mouse melanoma cells, Eur J Biochem 255, 139–146 (1998)
- DS Kim, SY Kim, JH Chung, KH Kim, HC Eun and KC Park, Delayed ERK activation by ceramide reduces melanin synthesis in human melanocytes, Cell Signal 14, 779–785 (2002)
- H Ando, A Itoh, Y Mishima and M Ichihashi, Correlation between the number of melanosomes, tyrosinase mRNA levels and tyrosinase activity in cultured murine melanoma cells in response to various melanogenesis regulatory agents, J Cell Physiol 163, 608–614 (1995)
- H Ando et al, Possible involvement of proteolytic degradation of tyrosinase in the regulatory effect of fatty acids in melanogenesis, J Lipids Res 40, 1312–1316 (1999)
- RE Hughes, Reduction of dehydroascorbic acid by animal tissues, Nature 203 1068-9 (1964)
- RW Scholz, KS Graham, E Gumpricht and CC Reddy, Mechanism of interaction of vitamin E and glutathione in the protection against membrane lipid peroxidation, Ann NY Acad Sci 570:514-7 (1989)
- Y Funasaka, AK Chakraborty, M Komoto, A Ohashi and M Ichihashi, The depigmenting effect of a-tocopheryl ferulate on human melanoma cells, Br J Dermatol 141, 20–29 (1999)
- A Slominski, D Tobin, S Shibahara and J Wortsman, Melanin pigmentation in mammalian skin and its hormonal regulation, Physiol Rev 84 1155–1228 (2004)
- P Downs et al, Modulating MC1R activity through the use of biomimetic peptides (2004)
- M Seiberg et al, The protease-activated receptor 2 regulates pigmentation via keratinocytes- melanocyte interactions, Exp Cell Res 254, 25–32 (2000b)
- M Seiberg, C Paine, E Sharlow, P Andrade- Gordon, M Costanzo, M Eisinger and SS Shapiro, Inhibition of melanosome transfer results in skin lightening, J Invest Dermatol 115 162–167 (2000a)
- L Minwalla et al, Inhibition of melanosome transfer from melanocytes to keratinocytes by lectins and neoglycoproteins in an in vitro model system, Pigment Cell Res 14, 185–194 (2001)
- EA Oliver, L Schwartz and LH Warren, Occupational leukoderma, Arch Derm and Syph 42 993 (1940)
- JP Ortonne and T Passeron, Melanin pigmentary disorders: Treatment update, Dermatol Clin 23 209–226 (2005)
- AD Katsambas and AJ Stratigos, Depigmenting and bleaching agents: Coping with hyperpigmentation, Clin Dermatol 19 483–488 (2001)
- K Maeda and M Fukuda, Arbutin: Mechanism of its depigmenting action in human melanocyte culture, J Pharmacol Exp Ther 276, 765–769 (1996)
- K Sugimoto, T Nishimura, K Nomura, K Sugimoto and T Kuriki, Synthesis of arbutin-a-glycosides and a comparison of their inhibitory effects with those of a-arbutin and arbutin on human tyrosinase, Chem Pharm Bull (Tokyo) 51 798–801 (2003)
- RE Boissy, M Visscher and MA DeLong, DeoxyArbutin: A novel reversible tyrosinase inhibitor with effective in vivo skin lightening potency, Exp Dermatol 8, 601–608 (2005)
- C Lin et al, Modulation of microphtalmia-associated transcription factor gene expression alter skin pigmentation, J Invest Dermatol 119, 1330–1340 (2002)
- C Saliou et al, Antioxidants modulate acture solar ultraviolet readiation induced NF-kappa- B activation in a human keratinocyte cell line, Free Radic Biol Med 26, 174–183 (1999)
- K Tsuji-Naito, T Hatani, T Okada and T Tehara, Evidence for covalent lipoyl adduction with dopaquinone following tyrosinase-catalyzed oxidation, Biochem Biophys Res Commun 343, 15–20 (2006)
- X Nair, P Parah, L Suhr and KM Tramposch, Combination of 4-hydroxyanisole and all trans retinoic acid produces synergistic skin depigmentation in swine, J Invest Dermatol 101, 145–149 (1993)
- C Cotelessa, K Peris, MT Onorati, MC Fargnoli and S Chimenti, The use of chemical agents in the treatment of different cutaneous hyperpigmentations, J Dermatol Surg 25, 450–454 (1999)
- W Smith, The effect of topical L(+)lactic acid and ascorbic acid on skin whitening, Int J Cosmet Sci 21, 33–40 (1999)
- I Kubo, I Kinst-Hori and Y Yokokawa, Tyrosinase inhibitors from Anacardium occidentale fruits, J Nat Prod 57, 545–551 (1994)