In recent years, several preservatives either have been banned or their use strongly limited, which is the case for formaldehyde, its releasers and isothiazolinones.1 In addition, some studies have misleadingly related parabens with a higher risk of cancer;2 so although parabens are the most commonly used preservatives in skin care due to their low sensitizing potential and good efficacy, with continued scrutiny from the market, many manufacturers are omitting them and promoting their cosmetics as paraben-free.
Currently, the ideal antimicrobial must show high antibacterial activity yet remain safe for human use and for the environment—and if possible, be based on naturally occurring substances. Considering these desired properties, the present paper explores ethyl lauroyl arginate HCl (LAE) for antimicrobial activity particularly against the microorganisms Malassezia furfur, involved in the proliferation of dandruff, and Propionibacterium acnes, involved in acne formation.
LAE Properties
LAE is a food-grade cationic surfactant with antimicrobial properties that is derived from the natural building blocks lauric acid and L-arginine (see Figure 1). In September 2005, LAE was Generally Recognized as Safe (GRAS) by the US Food and Drug Administration (FDA).3 In addition, it is a readily biodegradable material with low aquatic toxicity, it does not irritate the eyes or skin at recommended use levels, and it is non-sensitizing.4, 5
LAE was included in the European Union (EU) Directive 76/768/EEC (Annex VI)6 as an accepted preservative for cosmetic formulations with a maximum allowed dosage of 0.4% active. Furthermore, it was included in Annex III to the EU Directive as an allowable active for anti-dandruff shampoos, antibacterial soaps and deodorants at an upper limit of 0.8%.
The commercial product for cosmetic applications is a 20% solution of LAE in glycerina, which has been Ecocert- and Natrue-certified as 100% natural in origin and 100% naturally derived, respectively. Its natural origin makes it an attractive alternative to triclosan, which has caused concern due to its low biodegradability7 and antimicrobial resistance effects.8
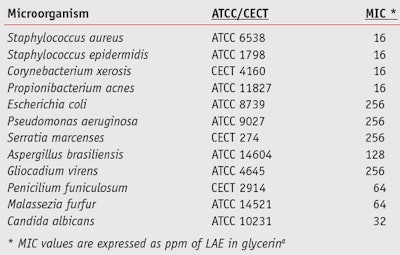
LAE inhibits the proliferation of microorganisms such as bacteria, fungi and yeasts by disturbing their cell membrane potential, altering cell permeability and thereby inducing the loss of cell viability.9, 10 Table 1 shows a list of minimum inhibitory concentrations (MIC) for LAE in glycerina against some representative microorganisms.
Due to its low MIC values for an array of microorganisms—i.e., bacteria, yeasts and molds,11, 12 LAE was considered for application as the sole preservative in cosmetic formulations. To evaluate its activity in anti-dandruff and dermopurifying applications, the authors performed suspension tests, described here, to compare several formulations in vitro. The efficacy of LAE in cosmetic formulations was then checked in vivo to corroborate these results.
While the activity of LAE was found to be non-pH dependent and stable from pH 3 to 7, the cationic nature of the molecule is responsible for some interactions with anionic raw materials, primarily in cleansing formulations or emulsions with high amounts of anionic thickeners. This interaction reduces the antimicrobial efficacy of LAE but it can be overcome by combining LAE with other antimicrobials to lessen this interaction and impart a synergistic effect.
Materials and Methods
Anti-dandruff: Currently, the most common strategy to fight dandruff is to remove scales to reduce the growth and adherence of Malassezia to the corneocytes13 using actives such as zinc pyrithione and piroctone olamine (see Figure 2). Zinc pyrithione is a powerful antifungal with some keratolytic properties, although it is water-insoluble and therefore must be incorporated into anti-dandruff shampoos or lotions as a suspension. It is also a compound with a certain degree of toxicity and has been described as damaging to the DNA of keratinocytes.14
Piroctone olamine is the ethanolamine salt of the hydroxamic acid derivative piroctone. Its antibacterial and antifungal properties also are applied in anti-dandruff applications, although it too is poorly soluble. While it is considered safer and less irritating than zinc pyrithione,15 zinc pyrithione generally has a better cost-efficacy ratio, which explains its widespread use.
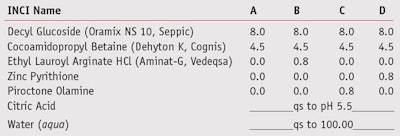
To compare the efficacy of these popular actives with that of LAE, shampoo formulations (see Table 2) were prepared including zinc pyrithioneb, salicylic acidc, piroctone olamined and LAEa. Xanthan gume was used in zinc pyrithione water solutions to keep the active ingredient in suspension, and all actives were tested against M. furfur (DSM 6170)f.
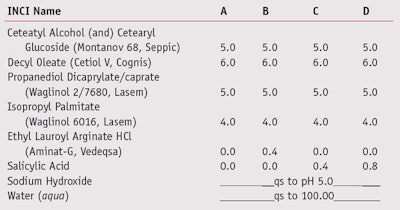
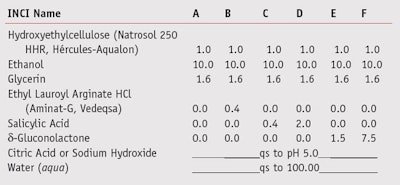
Anti-acne: In the case of acne, this condition is caused by, among other factors, increased sebum production and colonization of the follicle by P. acnes. Alpha hydroxyl acids (AHAs) such as δ-gluconolactoneg and organic acids including salicylic acid are the most commonly used materials to regulate sebum and provide mild antibacterial activity. Therefore, to compare the efficacy of these acids with that of LAE, several creams and gels (see Table 3) were developed incorporating salicylic acidc, δ-gluconolactoneg and LAEa; all formulas were then tested against P. acnes (ATCC 11827)h.
LAE inhibits the proliferation of microorganisms such as bacteria, fungi and yeasts by disturbing their cell membrane potential.
In vitro Suspension Tests
As noted, suspension tests16 were performed to assess the efficacy of the various actives. To test against M. furfur, the shampoo bases in Table 2 were prepared using Ecocert-certified surfactants and the test actives, each at 0.8%. Surfactants based on sodium laureth sulphate (SLES) were avoided due to the cationic nature of LAE.17 To evaluate activity against P. acnes, the formulations in Table 3 were developed, including a cream based on nonionic emulsifiers and a dermo-purifiying gel.
M. furfur was grown in potato dextrose agar (PDA) up to 108 Colony Forming Units (CFL) per mL. P. acnes was grown in trypticase soy broth up to 109 CFU per mL. Each test sample was inoculated with this culture at 1% (time zero), and an aliquot of 1 mL of each solution was removed at different times. A 0.1-mL sample of the appropriate dilution was spread onto PDA plates (for the fungi) or trypticase soy agar (TSA) plates in anaerobic conditions (for the bacteria) for enumeration using a spiral automatic spreaderj. Plates were incubated at 37 ± 2°C for at least 48 hr, after which they were read by an automatic colony counterk. The results were expressed as CFU per mL or gram of test sample.
In vivo Tests
Anti-dandruff and anti-acne in vivo studies were carried out for six weeks at EVIC Hispania based on several bibliographic references.
Anti-dandruff shampoos:18–20 Panelists (n = 20) used test shampoos A, B or C (see Table 2) twice every two days by massaging the scalp for one minute, rinsing and repeating the process. Note that the shampoo containing zinc pyrithione was excluded from the in vivo test due to its potential for skin irritation.
During the first two weeks, all volunteers used shampoo A without the anti-dandruff active; during the next four weeks, they used either shampoo B (n = 9) or shampoo C (n = 11). The state of the dandruff was evaluated by a dermatologist at the beginning of the study, after two weeks of pre-treatment, and after four weeks of treatment. A student’s t-test was used to determine the significance of differences measured.
Anti-acne creams and gels: Volunteers (n = 11) with greasy and acne-prone skin were chosen by a dermatologist21 to use test gel B (see Table 3) for 28 days by applying 1 mL of the product, measured with a syringe, all over the face twice daily, i.e., morning and night. Two parameters were evaluated on all panelists: pimple reduction and seboregulating effect, as measured with a sebumeterm.22
Results and Discussion: Anti-dandruff
Results of the suspension test for the four shampoo formulations against M. furfur are shown in Figure 3 and demonstrate the good activity of the LAE-containing formula (Shampoo B). This activity was comparable to the formula containing zinc pyrithione as the active (Shampoo D), and slightly superior to that of the shampoo containing piroctone olamine (Shampoo C).
To obtain in vivo results, a dermatologist evaluated the dandruff severity of the volunteers before and after the fourweek treatment with Shampoos B and C. The panelists who used both shampoos observed a significant reduction in dandruff (p < 0.05), as Figure 4a depicts. Among the reactive volunteers—i.e., those whose dandruff was reduced by more than 20%, improvement in their condition was more evident with Shampoo B than C.
Moreover, all panelists treated with Shampoo B were classified as reactive, as shown in Figure 4b. In Figure 5, it is possible to appreciate a significant and visible improvement in the scalp of a volunteer who used shampoo B for four weeks of treatment. The activity of LAE as an anti-dandruff agent was therefore validated in vivo and showed equal or superior performance to the classic anti-dandruff actives, with a better toxicological and eco-toxicological profile.4, 15
Results and Discussion: Anti-acne
Results of the suspension test for the creams in Table 3 on are shown in Figure 6, which reveals the antimicrobial activity of LAE as being superior to that of salicylic acid. In addition, Figure 7 shows the difference in activity between the gels in Table 4 incorporating LAE, salicylic acid or δ-gluconolactone. The quick-killing effect of LAE for the bacterium P. acnes is evident in the graph, in comparison with the slower effects of the other actives.
Preservation Challenge Testing
Following in vitro and in vivo testing, preservation challenge testing of the creams and gels was carried out following the CTFA Microbiology Guidelines23 and only Cream B was found to fulfill the preservation criteria without additional preservatives. This can be attributed to the ability of LAE to serve as both an active and a preservative. Similarly, this was the case with the dermo-purifying gels; only Gel B containing LAE as an active met the CTFA criteria for preservation.23
Gel B: In vivo
Considering the preservative challenge testing results, Gel B was chosen to conduct an additional in vivo test following the same protocol described to determine whether the activity observed corresponded to an improvement in acne. Figure 8 shows a significant reduction in the number of pimples for each panelist during the treatment with Gel B. Moreover, while not statistically significant, an important decrease in the average amount of sebum measured on volunteers was found (13.5% average decrease), indicating a sebolytic effect of the gel containing LAE as an active.
The quick-killing effect of LAE for the bacterium P. acnes is evident, in comparison with the slower effects of the other actives.
Conclusions
The natural-based antimicrobial LAE has been shown here, both in vitro and in vivo, to impart excellent activity as an anti-dandruff and dermo-purifying agent. Furthermore, its use as a food preservative and its regulatory approval as a preservative for cosmetic formulations reinforces the material’s safe standing. Taken together, the antimicrobial efficacy, natural origin and mild toxicological profile suggest LAE’s use as an alternative to traditional anti-dandruff and dermopurifying actives for personal care.