γ-Polyglutamic acid (γ-PGA) is a novel molecule that is a component of the mucilage of the fermented soybean food product commonly found in Japan called natto. The International Nomenclature Cosmetic Ingredient (INCI) name of γ-PGA is natto gum and it is classified as a film-forming agent. The sodium salt of γ-PGA, which is more commonly used and thus served as the test sample in this paper, displays the visual appearance of a sticky paste as illustrated in Figure 1.
The fermented soybean mucilage consists of a mixture of γ-PGA and fructan produced by Bacillus natto.1,2 Further research by Bovarnick revealed γ-PGA to be a fermentation byproduct freely secreted into growth medium outside of the cell walls of Bacillus subtilis.3 This discovery advanced collateral investigations to determine whether this bacteriological process could be reproduced in other species of Bacillus.4-11
Characteristics of γ-PGA
Recent work by Lung and Da-Yeh further illustrates the applications and production of γ-PGA from microorganisms by gamma irradiating to promote chemical cross-linking between monomeric units to form a γ-PGA hydrogel.12 The finished gel was lyophilized to assure its physical chemical stability.
The lyophilized product was characterized by Lung and Da-yeh by nuclear magnetic resonance (C13 and H1), Fourier Transform Infrared Spectroscopy and thermal gravimetric analysis in its protonated form, as well as the salts of Na+, K+, NH4+, Ca2+ and Mg2+.
The lyophilized product is a linear homopolypeptide consisting of only glutamic acid residuals composed of both dextro and levo optical isomers. The γ-peptide bond linkages exhibit a range of polymerization from 1000–12000 units. Figure 2 depicts the repeating units of γ-PGA.
The molecular weight ranged from 100,000 to 4,000,000 Daltons with an assay value above 95.0%. Physical-chemical properties such as the pH buffering capacity are exhibited in a range of 4.0 to 5.0.
Viscosity can vary as a function of temperature, pH and concentration to influence performance characteristics. The protonated form of γ-PGA is completely soluble in trifluorozincate, dimethyl sulfoxide, hydrazidine and a high concentration of sulfuric acid. The sodium form, which is soluble in water, can assume different molecular conformations depending on pH conditions and binding to other counter ions.
Figure 3 illustrates the elucidated structure of the protonated form of γ-PGA and Figure 4 depicts the elucidated structure of the sodium salt of γ-PGA.
The chemistry of γ-PGA allows it to be homogenously miscible as well as chemically stable in a matrix of ingredients typically used in facial creams, such as: glycerin, butylene glycol, alcohol, beta-glucan, hydrogenated lecithin, PEG-50, triethanolamine, disodium EDTA, methylparaben, ethylparaben, propylparaben, fragrance (parfum), vitamin E, carbomer, beta carotene, cetyl alcohol and disodium EDTA.
Applications in Cosmetics
Additional qualities of γ-PGA include the ability to form a smooth, elastic, self-moisturizing and soft film on the skin, resulting in improved sensory perception and protection of the outer layer of skin.13 It is an excellent hydrophilic humectant and is capable of increasing the production of such natural moisturizing factors as pyrrolidone carboxylic acid (PCA), lactic acid and urocanic acid, as compared to hyaluronic acid and soluble collagen.13 Additional work by Ichimara further supports these findings as illustrated in Figure 5.14
γ-PGA also offers antiaging benefits. A study conducted by Meiji Seika examined the ingredient’s potential as a natural replacement for collagen or hyaluronic acid.15 The origin of γ-PGA from fermented soybeans and its composition of enzymes, oligosaccharides and vitamins was shown in cosmetic formulations to favorably augment the natural moisturizing factor and elasticity of the skin more than collagen and hyaluronic acid during a 28-day period, as detailed in Figure 6. The study suggests that γ-PGA could successfully replace collagen and hyaluronic acid in skin care products.
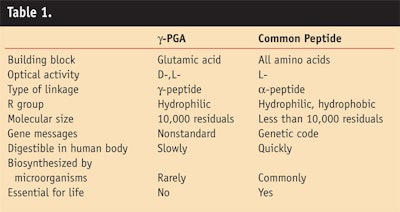
As Figure 6 demonstrates, products containing γ-PGA reinforce the skin's support structure to energize cells, stimulating the production of lipids and renewal of the epidermis. It has been shown to replenish and nourish the skin, leaving it smoother and plumper; it also is nontoxic to humans, natural and environmentally friendly. The general range of use level is from 0.3–2.5%, depending on the product form being deployed for end product use. The acute oral toxicity test for γ-PGA in the form of its sodium salt gave an LD50 > 5,000 mg/kg/day. Biodegradability tests of the sodium salt of γ-PGA revealed that 90% degraded in 180 days. Table 1 compares characteristics of γ-PGA to other peptides.
γ-PGA contains a robust amount of vitamins, oligiosaccharides and beneficial enzymes currently not found in the nonfermented soybean product.12 It can be described as a white to off-white, granulated, free-flowing powder. Purity by HPLC is a minimum of 70%, and the minimum assay value determined by gravimetric analysis of the salt of the γ-PGA is no less than 90% wt/wt. The loss on drying is no more than 10% wt/wt γ-PGA. The heavy metals composition is no more than 0.002% and the total bacterial count test for microorganisms is not greater than 500 CFU/g for a cosmetic-grade material. Salmonella and Escherichia coli are absent in γ-PGA. Its particle size is 100% through 100 mesh screen.
γ-PGA Hydrogel
γ-PGA hydrogel is another form of γ-PGA. It is produced by gamma irradiating PGA to promote chemical cross-linking between monomeric units. Microscopic examination of the γ-PGA hydrogel reveals a multi-baglike structure that is capable of enhanced absorption of moisture because of the cross-linking of the peptides (see Figure 7).16–18
The physical-chemical properties of the hydrogel enable it to absorb moisture 5,000 times its own weight.16,17 The amount of water contained within the γ-PGA hydrogel is influenced by pH and salt content. The specific water contents and degree of swelling markedly decrease with the addition of electrolytes and pH adjustment to an acidic environment because of the conformational changes that the molecule undergoes in acidic pH. This allows cosmetic formulators to enhance the moisturizing ability of hair or skin formulations with the addition of the proper amount of electrolytes and appropriate pH adjustments. The release of moisture forms a film of protection, which is important in applications such as facial masks that are used to hydrate the skin as well as reduce the appearance of wrinkles. The conceptual before-and-after effects of the γ-PGA hydrogel on the natural moisturizing factor of the skin are depicted in Figure 8.
The moisture loss of the skin was significantly reduced with the use of γ-PGA, compared to its absence. Figure 9 shows the effects of γ-PGA on transepidermal water loss over a 3 hr time period.19
Equivalency studies20 were performed against glyercol to examine moisturizing efficiency and water retention on the IMS membrane. The results shown in Figure 10 reveal that lower concentrations of PGA hydrogel yielded approximately the same results of water retention as higher concentrations of glycerol. This observation reveals that the hydrogel has potential as an alternative to glycerol in cosmetic formulations.
Hair Applications
In addition to skin care, research20 has shown that γ-PGA can be used as an additive to increase hair strength to better withstand the bleaching process. The material maintains hair strength by increasing its natural moisture retention ability and forming a protective layer on the hair.
It also exhibits the ability to release moisture within the hair formulation to form a barrier capable of diluting the chemical interactions of the applied colorings with the protein makeup of the hair. This diluting action helps to maintain the strength of hair.
The layer formed by γ-PGA was also found to protect hair from damage incurred by bleaching. Investigations were continued to study the effects of the material on maintaining hair strength and preventing hair damage due to bleaching processes (see Figure 11).20
Electron microscopy images were taken20 (see Figure 12) and detail the differences in physical appearance of hair treated with and without γ-PGA.
Treatments exposed the hair to 20 min of bleaching for coloring purposes. A 1.5% sample incorporating γ-PGA was added in the bleaching products to help diminish the damage caused to hair.20
Conclusion
The use of γ-PGA as a value-added component to enhance the properties of existing or novel personal care products as a moisturizer, exfoliant and wrinkle-remover should be investigated through laboratory studies to optimize product development.
By understanding the physicochemical properties of γ-PGA, formulators can develop cosmetic products capable of being both a moisturizer and an exfoliant. The insight gained from the application of y-PGA could enable formulators to reinvigorate products lacking these attributes. The industry as a whole can benefit from the use of y-PGA as a low cost, environmentally friendly component with wide ranging applications.
References
1. S Sawamura, Bacillus natto, J Coll Agri Tokyo 5 189–191 (1913)
2. H Fujii, The formation of mucilage by bacillus natto, part III: Chemical constitutions of mucilage in natto (1), Nippon Nogeikagaku Kaishi 37 407–411 (1963)
3. M Bovarnick, The formation of extracellular D(-)glutamic acid polypeptide by bacillus subtillus, J Biol Chem 145 415–424 (1942)
4. C Cheng, Y Asada and T Aaida, Production of y-polyglutamic acid by bacillus subtillus A35 under denitrifying conditions, Agric Biol Chem 53 2369–2375 (1989)
5. A Goto and M Kunioka, Biosynthesis and hydrolysis of poly γ-glutamic acid from bacillus subtillus IFO3335, Biosc Biotechnol Biochem 56 1031–1035 (1992)
6. T Hara and S Ueda, Regulation of polyglutamate production in bacillus subtillus (natto): Transformation of high PGA productivity, Agric Biol Chem 46 2275–2281 (1982)
7. RD Housewright, The biosynthesis of homopolymeric peptides in The bacteria: A treatise on structure and function, vol III, eds IC Gunsalus and RY Stanier, Academic Press, New York, 389–412 (1962)
8. H Kubota, Y Nambu and T Endo, Convenient and quantitative esterification of poly (γ-glutamic acid) produced by microorganism, J Polym Sci Part A: Polym Chem 31 2877–2878 (1993)
9. S Maurao, The polyglutamic acid fermentation, Koubunshi 16 1204–1212 (1969)
10. CB Thorne, CG Gomez, HE Noyes and RD Housewright, Transamination of D-amino acids by bacillus subtillus, J Bacteriol 68 307–315 (1954)
11. FA Troy, Chemistry and biosynthesis of the poly (γ-D glutamyl) capsule in bacillus lichenformis. 1. Properties of the membrane mediated biosynthetic reaction, J Biol Chem 248, 305–316 (1973)
12. IL Lung and YT Van, Da-Yeh University, Taiwan, Bioresource Technol 79 207–225 (April 2001)
13. JP Patent 11240827, Preparation composition for external use containing gamma polyglutamic acid and vegetable extract in combination, issued to K Hasebe and M Inagaki (1999)
14. Increase of NMF in the stratum corneum by y-PGA, HA and soluble collagen, data provided by Ichimaru, Japan (2002)
15. Comparison table of the effectiveness of Y-PGA, HA and Soluble collagen in cosmetic formulations, data provided by Meiji Seika, Japan (2003)
16. M Kunioka, Properties of hydrogels prepared by irradiation in microbial poly (γ-glutamic acid) aqueous solutions, Kobunshi Ronbunshu 50 755–760 (1993)
17. HJ Choi and M Kunioka, Preparation conditions and swelling equilibria of hydrogel prepared by γ-irradiation from microbial poly γ-glutamic acid, Radiat Phys Chem 46 175–179 (1995)
18. HJ Choi and M Kunioka, Synthesis and characterization of pH sensitive and biodegradeable hydrogels prepared by γ-irradiation using microbial poly γ-glutamic acid and poly lysine, J Appl Polym Sci 58 807–814 (1995)
19. Data collected from Providence University, Taiwan (2001)
20. Data collected by Ichimaru, Japan (2003)