Microbeads are often made from synthetic plastics of various chemistries fabricated in different sizes, shapes, and densities depending on their intended application. There is wide variety in the types of chemistries encountered for a microplastic bead, including polyethylene (PE), polyethylene terephthalate, polypropylene, polyamide, polyesters, polystyrene and polyvinyl chloride.1 However, PE is the highest volume commodity plastic offered globally and is the primary plastic used for manufacturing microbeads.2, 3 PE microbeads also made up a majority of the beads used within the personal care industry but these are quickly being phased out for alternate, non-PE abrasives.3
Microbeads had been used within a variety of personal care compositions to assist with exfoliation due to their abrasive nature, and for the delivery of skin care benefits such as acne treatments, deep cleansing, skin brightening and smoothing, and evenness of skin tone. Generally, these compositions are applied regularly and over extended time periods to obtain the most efficacy. The properties of the microbead, such as the particle size, density, morphology and hardness, are important for abrasive and sensory attributes. Frequent usage is encouraged through desirable product esthetics and sensation during application, which also provides some indication that the product is having its intended effect.
PE Chemistry and Manufacturing
Polyethylene is classified as a polyolefin, which is derived from the catalyzed polymerization of the alkene monomer ethylene.4 PE may demonstrate a dramatic range of physical properties depending on the synthetic route used to create it; its general categories include low density polyethylene (LDPE), linear low density polyethylene (LLDPE) and high density polyethylene (HDPE).
LDPE is more widely employed and is typically synthesized using free radical initiators, while LLDPE and HDPE are generally made using coordination catalysts, such as Ziegler-Natta or Philips.5, 6 In contrast to LDPE, HDPE and LLDPE are produced via copolymerization of a-olefins reacted as co-monomers and will result in different structures, as seen in Figure 1.7–9
With respect to HDPE and LLDPE, the choice of catalysts can aid in controlling the material’s linearity and tacticity, which can impact the molecular weight, its distribution, and microstructure crystallinity—observed on the macroscopic scale as differences in material rigidity, density and tensile strength. The presence of branching and placement of branches within the PE microstructure will also be affected by the catalyst used.10, 11
The amount of short-chain and long-chain branching within the PE structure will also affect the material properties impacting the microstructure arrangement and level of crystallinity. Variations in material performance and application will be further affected by catalyst choice, synthesis vs. processing conditions, additives and fillers.
PE microbeads have proven to be advantageous for use in personal care because of their well-defined material synthesis and customizable performance based on their chemistry. Since PEs have a reliable synthetic pathway for predictable properties, formulators can achieve consistent visual appeal and color, controlled abrasiveness, particle morphology and density, as well as chemical inertness and cost-effective production with a robust material supply.
Further, based on the chemical composition of PE, the material is resilient in pH extremes, high-electrolyte and high-surfactant environments, resisting color loss and material degradation over a product’s shelf-life. Finally, the strong demand for polyethylene also generally sustains high production volumes, enabling efficient large-scale production at a relatively low cost.12
Despite the benefits realized by consumer goods manufacturers and end users, however, PE microbeads have concerned regulators, which has translated to a public discussion.
Variations in polyethylene performance will be affected by catalyst choice, synthesis vs. processing conditions, additives and fillers.
PE Microbeads: Public Discussion
The discussion over PE microbeads has become more urgent with the passage by the U.S. Congress of the Microbead-Free Waters Act in December 2015. The law will effectively ban the sale of rinse-off cosmetics containing intentionally added plastic microbeads beginning Jan. 1, 2018 and currently bans the manufacture of these cosmetics, which started July 1, 2017.13
Europe also has taken notice, with many individual member companies of Cosmetics Europe publicly stating they will discontinue plastic microbead use in cosmetics, which may potentially end up in the aquatic environment and for which alternatives exist.3 Furthermore, on Oct. 21, 2015, Cosmetics Europe recommended its members discontinue using PE microbead-containing wash-off cosmetics placed on the market as of 2020. This move engages the whole of Cosmetics Europe membership and facilitates sector-wide best practices.14–16
Microplastics Beyond Cosmetics
Although personal care products have been the focus of discussion for PE microbeads, microplastics can be generated from many other industrial sectors. Indeed, microplastics may form through the conversion of microplastics from PE origin or other types of plastics, as noted by the Personal Care Products Council in Understanding Microplastic Litter.17 Such larger plastics may be introduced into the aquatic environment and degraded down to the particle size defined by the Microbead-Free Waters Act.13 This conversion of larger plastics into smaller plastics is sometimes referred to as the formation of secondary microplastics.
This distinction in the origin of microplastics is seldom articulated when confronting the issue. Formal studies on the contribution of the personal care industry toward accumulation are complicated due to contributions from secondary microplastics; however, the levels introduced by personal care products are estimated to be a fraction of the total possible from all sources.18
Ensuing legislation has necessitated the removal of PE plastic microbeads and a subsequent search for non-plastic alternatives for product formulations. Within the personal care industry, several major corporations have led the move toward microplastics removal from their product portfolios. Johnson & Johnson was one of the first to publicly communicate, in 2013, the total removal of plastic microbeads from its global personal care product offerings by the end of 2017. Other companies followed; L’Oréal pledged plastic microbead removal from its scrub products by 2017, as well as several other companies including, but not limited to, Unilever, Procter & Gamble, Colgate-Palmolive and Lush.19–21
PE Microbead Removal and Screening for Alternates
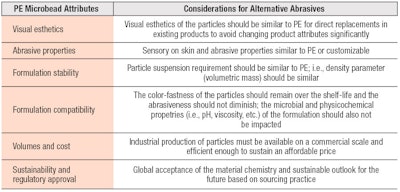
The preliminary screening process for material candidates must consider supply chain, technical viability and consumer acceptance to lead to a sustainable option (see Figure 2 and Table 1). Choosing a suitable alternative will be based on the formulary requirements, as well as the consumer expectations for that product. Larger particle sizes may provide a coarser exfoliating experience, while smaller particles may provide milder abrasiveness for sensitive skin.
Products with alternate options must be esthetically desirable. They must also exhibit robust stability and skin benefit claims for smoothness, softer skin or the avoidance of micro lacerations must be substantiated. The supply chain may also be limited for certain alternatives depending on their feedstock and origin, which can be problematic for business continuity plans.
Several personal care companies are exploring alternative exfoliants to common PE microbeads;19 for example, Lush offers adzuki beans, ground almonds, sea salt, mica and minerals. L’Oréal has focused on clays and powdered fruit kernels. Beiersdorf AG has explored the use of cellulose, hydrogenated castor oil and hydrated silica; and Unilever is targeting apricot kernels, cornmeal, ground pumice, silica and walnut shells.22-24
Although a variety of alternatives exists, their practicality and effects in formulations must be tested before implementing them. Careful consideration must also be given to natural material replacements such as nutshell powders for exfoliation, as these may be allergenic to a segment of the population and could affect product safety and claims.
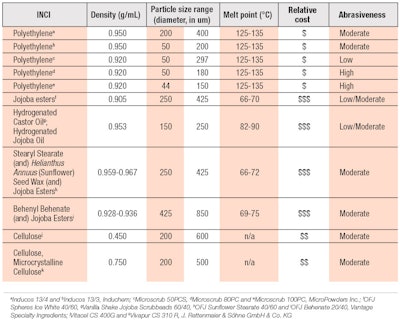
Water-soluble alternatives: Several characteristics associated with PE microbeads should be considered when examining natural alternative exfoliants as replacements (see Table 2). Factors such as sustainability, visual color, suspension requirements, abrasiveness and inertness with respect to the formulation composition and environment may be important for the product. Water-soluble alternatives are degradable and may be customized for abrasiveness similar to PE. Some examples are sugar and sea salt abrasives, which degrade quickly in water and are available in large volumes. These exfoliants may range from fine to coarse granules to meet the application requirements and, due to their water-solubility, they may dissolve upon application, providing a different sensory experience than water-insoluble alternatives.
However, with water-soluble alternatives, the formulator must understand how to enable the material to remain insoluble in the product base to deliver the performance of an abrasive exfoliant. The formulations developed may need to be anhydrous or supersaturated to achieve a stable shelf-life for the exfoliant and to ensure an efficacious benefit is delivered.25
Water-insoluble alternatives: Insoluble alternatives derived from natural sources are also available and provide added formulation flexibility as they are not limited by water-solubility. Polylactic acid (PLA), for example, is an aliphatic natural polyester produced from the polymerization of lactic acid. This process may be carried out through microbial fermentation of carbohydrates producing lactic acid that is recovered, dehydrated and polymerized to create PLA. Here, the carbohydrate source is generally selected based on price and can vary from corn starch and molasses, to sugar cane and rice. Additional factors affecting the feedstock choice are the availability, costs of lactic acid recovery and purification.26
To replace PE, consider the lubricity, particle size distribution, surface roughness, contact angle, melting point and density of alternates.
PLA has gentle abrasive properties and adequate morphology and density compared with PE microbeads.27, 28 This material is also available at an industrial scale and offered by suppliers as a cost-effective renewable option. However, product stability may have associated challenges. Depending on the formula environment, additional optimization of formulas may be required since PLA beads potentially discolor over time and lower the product pH due to release of lactic acid.12, 27 Legal challenges may also arise from the use of PLA as this is considered a plastic, though derived from a variety of natural sources.
Plant-derived options: Other alternative candidates for exfoliation may be plant-derived, from natural sources. These alternative abrasives, with the right physical morphology and chemistry, can be formulated in skin care bases to provide stable products that are esthetically pleasing, efficacious and provide consumer-perceptible skin care benefits.
Cellulose exfoliating abrasives, for example, are derived from the tough fibers originating in wood and plants. These cellulose materials, however, have been precluded from a large range of applications due to low porosity and their non-spherical shape.29, 30
There are three main steps to making cellulose exfoliants: 1) liquefaction of the cellulose; 2) dispersion of the cellulose phase in immiscible liquid; and 3) solidification and recovery of the particle. Novel processes are also being employed by an extrusion method through small holes into tubular membranes, which create more spherical droplets that can be removed from the membrane using vegetable oil. These are then collected, set and separated from the oil before use. Such exfoliating abrasives can be further altered for applications through modifications in their structure to adjust hardness.30
Jojoba is another alternative, derived from the jojoba seed through pressing or extraction of the oil, which is a liquid wax ester. The particle may be further refined through hydrogenation to increase hardness and shaped into small spheres that function as exfoliators, which can be controlled for size and roughness.31, 32 Alterations can be made to jojoba exfoliants by pairing them with different oils and esters to refine their properties. These abrasive properties are robust enough to remain stable during processing at 55-60°C, depending on the derivative, and in high electrolyte surfactant based systems.32 A general comparison of PE microbeads with jojoba and cellulose alternative abrasives can be made by microscopy to understand potential morphology differences (see Figure 3). These alternatives are available in spherical as well as angular morphologies for a range of applications.
Conclusions
With legislation and consumer preferences turning manufacturers away from PE microbeads, formulators can adapt by using alternative abrasives; however, when choosing, it is important to review and compare the ingredient chemistries, particle morphologies and physical properties of the various bead types. The lubricity, particle size distribution, surface roughness, contact angle, melting point and density also should be considered—along with formulation aspects such as compatibility, stability, esthetics and efficacy, which are key factors for success.
Balancing the aspects of surface roughness, particle size, shape and hardness, formulators can create a similar exfoliation experience to a synthetic based powder, which may be further customized for specific abrasiveness and sensorial experiences. PE microbead alternates that are soft to moderately hard may be used in facial products, while more abrasive particles are applied in body products. Even harder, more abrasive alternatives are employed for use on the feet or hands.26, 33 Further comparisons of the differences between commonly used PE microbeads and natural abrasive alternatives are shown in Table 2. Finally, consider their potential combination to meet formulary requirements and costs.
References
All websites accessed Oct 11, 2017.
- Microbeads—A science summary, Environment Canada (2012) pp 4-28
- AL Andrady, The plastic in microplastics, Marine Pollution Bulletin 119 (2017) pp 12-22
- http://blog.euromonitor.com/2016/10/plastic-not-fantastic-industry-responds-to-us-microbeads-ban.html
- Final report on safety assessment of polyethylene, Intl J Toxicology 26 (supplement 1) 115–127 (2007)
- JV Gruber, Elements of polymer science, Principles of Polymer Science 25-33 (1999)
- AL Andrady, Microplastics in the marine environment, Marine Pollution Bulletin 62 1596-1605 (2011)
- YV Kissin, Linear low density polyethylene, in K Othmer, Encyclopedia of Chemical Technology, published online, John Wiley & Sons, Hoboken, NJ (Apr 24, 2015) pp 1-32
- E Benham and M McDaniel, High density polyethylene, in K Othmer, Encyclopedia of Chemical Technology, published online, John Wiley & Sons, Hoboken, NJ (May 13, 2005)
- N Maraschin, Low density polyethylene, in K Othmer, Encyclopedia of Chemical Technology, published online, John Wiley & Sons, Hoboken, NJ, volume 0 pp 1-40
- J Pritchard, Introduction to polyolefins, in Polyolefin Reaction Engineering First Edition (2012) pp 1-14
- M Cole, TS Galloway, C Halsband and P Lindeque, Microplastics as contaminants in the marine environment: A review, Marine Pollution Bulletin 62 2588-2597 (2011)
- CH Morice, Cellulose acetate technology to replace banned PE beads, HPC Today 10(5) 57-60 (2015)
- www.congress.gov/bill/114th-congress/house-bill/1321/text
- http://cosmetics.specialchem.com/news/industry-news/cosmetics-europe-reduction-plastic-microbeads-000186560
- www.cosmeticsandtoiletries.com/regulatory/region/northamerica/Canada-Lists-Microbeads-as-Toxic-Paves-Way-to-Ban--385038371.html
- www.cosmeticsandtoiletries.com/regulatory/region/europe/CIDESCO-Supports-Microbead-Ban-Calls-for-Safe-Alternatives-320492052.html
- http://multibriefs.com/briefs/council/microplastic.jpg
- Ibid Ref 17
- https://uk.lush.com/article/bead-different-natural-alternatives-microplastics
- http://us.pg.com/our-brands/product-safety/ingredient-safety/microbeads
- www.safetyandcarecommitment.com/Ingredients/Microbeads
- http://blog.euromonitor.com/2016/10/plastic-not-fantastic-industry-responds-to-us-microbeads-ban.html
- www.loreal.com/media/news/2017/mar/reformulation-of-products-using-microbeads
- www.unilever.com/sustainable-living/what-matters-to-you/micro-plastics.html
- www.cosmeticsandtoiletries.com/research/methodsprocesses/2671356.html
- https://knowledge.ulprospector.com/369/
- T Ghaffar et al, Recent trends in lactic acid biotechnology: A brief review on production to purification, J Radiation Res and Applied Sciences 7(2) 222-229 (2014)
- P Gemeiner, MJ Benes and J Stamberg, Biotechnology Advances 30 321-328 (2012)
- P Gemeiner, MJ Benes and J Stamberg, Bead cellulose and its use in biochemistry and biotechnology, Chem Papers 43(6) 805-848 (1989)
- www.cosmeticsdesign-europe.com/Formulation-Science/Is-cellulose-the-answer-to-eco-friendly-microbeads
- http://kosterkeunen.eu/eu/home
- www.vantagespecialties.com/ pccfindingalternativessyntheticexfoliatingbeads/
- HA Leslie, Review of microplastics in cosmetics (R14/29), Institute for Environmental Studies, University of Amsterdam (2014)