Previous articles in this series have focused on measuring changes in the properties of hair as a function of treatment with conventional daily-use products—where efficacy arises from the ability to alter surface properties. For example, it has been shown how, in technical terms, conditioning products deliver surface lubrication to facilitate manageability and mediate degrading sensorial properties;1 styling products induce interactions between neighboring fiber surfaces to improve the ability to both create and maintain styles;2, 3 while shampoos subsequently clean the hair surface of all these deposits and other exogenous soils.
The benefits of these products are undeniable. At the same time, however, certain other hair-related attributes associate with the internal structure—the cortex—of fibers, and consequently are unaffected by these treatments. The primary ingredients in conventional, daily-use formulations are surfactants, polymers, oils, etc., all of which are somewhat sizeable and seemingly restricted from appreciably penetrating into hair.
Following this thought process, the suggestion could be made that a new and different class of hair care products could be developed—products that specifically seek to alter hair’s internal properties. Such products must seemingly rely on the activity of smaller molecules that have improved potential for fiber penetration.
Currently, such products involve reactive chemistry, which also causes damage to the hair structure. Yet, there may be the potential for manipulation by non-reactive routes. In any case, activity in such products must begin with appreciable diffusion of molecules into hair; and it is surprising how little is known about this fundamental topic.
This article focuses on understanding and quantifying the penetration of reactive ingredients into hair—specifically, perm actives. A second article in this series will address diffusion of non-reactive molecules into hair and their subsequent ability to manipulate internal properties.
Perm Chemistry
The market for perms has been soft for many years as straight hair styles have dominated. Yet perm chemistry arguably still represents the best option for permanently changing the shape of hair—making the straightening of curly hair equally as easy as creating curls in straight hair.
The hair straightening market has been shaken up in recent years by so-called Brazilian Straightening treatments, which are highly effective but utilize formaldehyde as an active. Nonetheless, the hullabaloo created by these products has prompted companies to re-examine routes for changing hair shape—and many have returned to exploring conventional perm chemistry.
As a chemist, it is gratifying for me to recognize that the perming process uses straightforward textbook chemistry. Equation 1 shows the typically quoted reaction scheme, where a thiol (RSH) cleaves cystine disulfide bonds within the hair’s keratin protein (K-S-S-K) to produce a mixed disulfide (K-S-S-R) and cysteine (sometime called ½ cystine, HS-K).
K-S-S-K + RSH ↔ K-S-S-R + HS-K
Eq. 1
K-S-S-R + RSH ↔ 2K-SH + RSSR
Eq. 2
The continued reaction of the thiol with the mixed disulfide is also possible (see Equation 2), with the production of another mole of cysteine and the dimer of the original thiol (RSSR). Manipulation is achieved by deconstructing a portion of the hair’s internal structure, followed by subsequent reformation with the hair anchored in a new desired configuration.
In actuality, it is widely acknowledged that the active species in the perm reaction is the thiolate ion (RS–) as opposed to the thiol itself. Therefore the first step in the above reaction scheme involves de-protonation of the thiol, as illustrated in Equation 3. This equation also explains the considerable pH dependence of the perm reaction, in that higher pH levels lead to the increased concentration of the active thiolate ion.
RSH ↔ RS– + H+
Eq. 3
It now becomes evident that appropriate balancing of these equations must also involve electronic charge, in addition to atoms. Electrons must be added to the left-hand side of an equation for disulfide bond cleavage (Equation 4), and to the right-hand side for the conversion of the thiol to its dimer (Equation 5) to yield the appropriately balanced total ionic Equation 6.
K-S-S-K + 2e– ↔ 2 KS–
Eq. 4
2RS– ↔ R-S-S-R + 2e–
Eq. 5
K-S-S-K + 2RS– ↔ 2KS– + R-S-S-R
Eq. 6
In accordance with standard textbook chemistry, the acquisition of electrons represents a reduction reaction, while the loss of electrons is oxidation. Therefore, cleavage of disulfide bonds within the hair represents a redox reaction: the cystine bonds are reduced; the thiol is the reducing agent and is itself oxidized in the process.
The reversal of this process to reform keratin disulfide bonds necessitates an oxidative second treatment. This process is usually accomplished using hydrogen peroxide (see Equation 7).
2K-SH + H2O2 ↔ K-S-S-K + 2H2O
Eq. 7
Equations in chemistry textbooks generally relate to reactions taking place in gaseous or liquid states where reactants readily come into contact. The complicating factor in the perm process involves one reactant being inside hair’s complex structure, while the other begins inside a product package.
Penetration into Hair
Conjecture about an ingredient’s ability to penetrate into hair to produce some benefit is common, yet research suggests diffusion into hair is not a trivial task. It appears likely that the more widely-heard opinion stems from the ease by which water is able to penetrate into hair.
Water is an especially small molecule with the additional ability to disrupt electrostatic bonding within hair. Its infiltration also causes fibers to swell, which further facilitates the diffusion process. It may be speculated that dissolved materials would readily be carried into hair along with their solvent, but this does not appear to be entirely true.
By means of analogy, the discipline of liquid chromatography is invoked, where a solvent readily passes along a stationary column with dissolved materials traversing at differing rates, depending on factors such as size and chemical interactions. In the same way, to access the hair bulk, water-soluble materials must permeate through a protein structure, which presents a variety of options for interactions with the solute. Our own studies are suggesting sizable differences in the ability for even relatively small molecules to penetrate hair (more on this in the upcoming second article).
In any complex process where multiple factors are involved, progress can only proceed at a rate that is dictated by the slowest step involved (i.e., the limiting step). In terms of the perm process, if the reaction rate is fast but diffusion is slow, then diffusion-limited conditions are encountered.
In theory, this dictates the presence of an advancing reaction “front” within fibers, where unreacted cystine bonds lie ahead and reduced bonds are found behind. Conversely, fast diffusion and slow reaction leads to reaction-limited conditions, where the active readily penetrates throughout the hair before appreciable reaction takes place. Accordingly, there would be no well-defined interface.
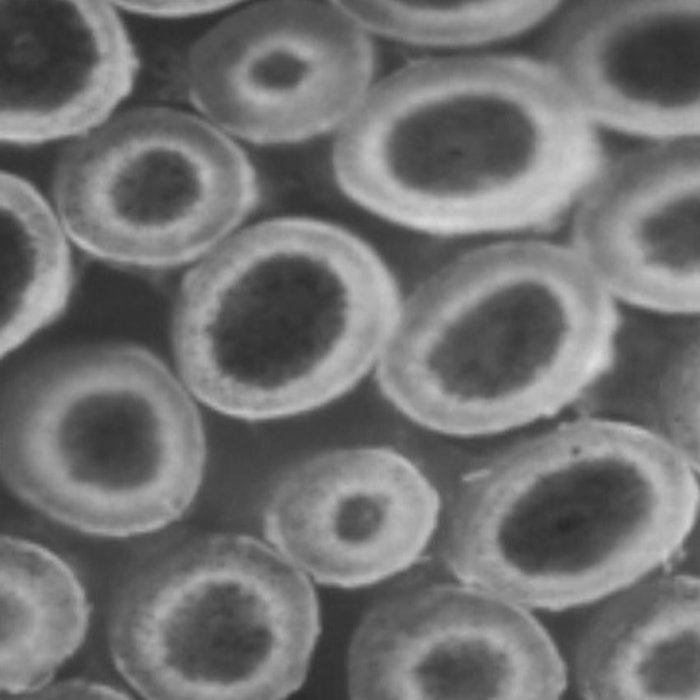
Both of these behaviors have been encountered and visualized experimentally by means of microscopy combined with staining techniques.4 That is, freshly permed hair is treated with reagents that specifically adhere to free thiol sites to indicate where disulfide bonds have been broken. Figures 1 and 2 show examples of these two behaviors.
Single Fiber Tensile Kinetics
Early efforts to follow the rate of the perming process involved the slow and tedious approach of chemically analyzing cystine content after exposing hair to reagents for differing periods of time.5 An interesting alternate proposition was suggested in 1950 by Reese and Eyring.6 The tensile properties of hair fibers should progressively decrease upon soaking in perm solution as strength-supporting cystine disulfide bonds are constantly being cleaved. There is, therefore, the potential to use changes in tensile properties of individual hair fibers with time as a proxy for reaction progression.
This approach was subsequently popularized in perm research during the 1980s and 1990s by Wickett,7-12 who also coined the phrase Single Fiber Tensile Kinetics (SFTK). Figure 3 shows a custom-built SFTK test cell that attaches to the base of a commercial tensile testing device. Individual hair fibers are carefully glued between plastic tabs such that the test specimen measures 2 in in length. Prior to gluing, a punch is used to produce a precisely placed hole at the center of each plastic tab, proving a reproducible means of anchoring test samples.
The cell contains a hook in the base for anchoring one end of the fiber, while a second hook attaches to the instrument’s load cell. Wickett’s approach involved applying a static 2% strain to the hair and monitoring the decrease in stress as a function of time. The present author later advocated cycling a 2% intermittent strain4 at 30-sec intervals to track the progressive decrease in tensile properties.
Testing involves first pre-straining the individual fibers in water to yield baseline tensile properties. The water is then quickly drained via the faucet on the front of the cell and rapidly replaced with the perm test solution. The mechanical straining process begins immediately; Figure 4 shows a typical representation of experimental data.
A number of assumptions must be made in directly equating changes in tensile properties to the reaction progression; many of them appear distinctly dubious. However, our validation studies showed remarkably reproducible results that consistently showed predictable changes to systematic alterations in experimental parameters.
The Effect of Hair Type
Hair samples and tresses provided by various companies for in vitro experiments usually consist of blended hair that is obtained from a selection of individuals.13 Initial SFTK testing involving this hair source yielded a high standard deviation and poor reproducibility but this issue was overcome by switching to single-source hair.
At the same time, the new results consistently demonstrated sizable differences in stress relaxation rates for samples obtained from different individuals when the hair came into contact with a common perm solution. This finding is in line with real-life experience, where it has long been known that a given perm solution could produce the desired result on one individual while being wholly ineffective on the next.
The poor response of “resistant hair” to the perming process is theorized to be a consequence of slower transformation rates. These slower rates insufficiently break the disulfide bonds during a given exposure time to the thiol solution. Nonetheless, nothing in our current knowledge of hair explains why these perming rates should be so different.
The concept of “resistant hair” is not restricted to perms; it is also recognized in bleaching and coloring hair. This seems to shifts the reason for this occurrence from the perm reaction to the hair itself. One possible explanation could involve differing diffusion rates in hair from different individuals.
We are currently investigating this hypothesis by exploring if there are differences in swelling rates of hair from different individuals. If so, correlations to SFTK results will be sought.
Summary
Salts of thioglycolic acid have remained the most popular perm “active” since the inception of cold wave treatments in the early 1940s. Hopefully this summary explains the attraction for this material. Its technical efficacy lies with a suitably high oxidation potential and the ability to refine its efficacy via both concentration and pH. Yet, this is also a relatively small molecule, which presumably is equally critical in allowing appreciable penetration into the hair such that it can react with structure-supporting chemical bonds. Likewise, alternative shape-shifting hair treatments use other small reactive molecules; e.g., formaldehyde in Brazilian Keratin treatments and the hydroxide ion in high-pH Ethnic relaxer products.
Single fiber kinetic analysis experiments consistently and reproducibly show significant variations in the reactivity of hair from different individuals with a given perm solution. While nothing currently can explain this occurrence, perhaps the most instinctive causation involves non-uniformity in the diffusion rates of materials into hair of different individuals. If this is so, then the already complex topic of materials accessing hair’s interior becomes more complicated, still.
The next article in this series will examine the penetration of non-reactive molecules into hair and their subsequent effect on fiber properties.
Acknowledgements: Figures 1 and 2 were reproduced with the permission of the Journal of Cosmetic Science.
References
- TA Evans, Cosm & Toil 126(8) 558-563 (Aug 2011)
- TA Evans, Cosm & Toil 29(5) 46-49 (Jun 2014)
- TA Evans, Cosm & Toil 129(7) 42-46 (Sep 2014)
- TA Evans, TN Ventura and AB Wayne, J Cosmet Sci 45 279-298 (1994)
- JB Speakman, J Soc Dyers Col 52 335-346 (1936)
- CE Reese and H Eyring, Textile Res J 20 743-750 (1950)
- RR Wickett, J Cosmet Sci 34 301-316 (1983)
- RR Wickett and BG Barman, J Cosmet Sci 36 75-86 (1985)
- RR Wickett and R Mermelstein, J Cosmet Sci 37 461-473 (1986)
- RR Wickett, Cosm & Toil 106 37-47 (1991)
- MA Manuszak, ET Borish and RR Wickett, J Cosmet Sci 47 49-58 (1996)
- MA Manuszak, ET Borish and RR Wickett, J Cosmet Sci 47 213-227 (1996)
- www.cosmeticsandtoiletries.com/testing/efficacyclaims/Hair-as-a-Test-Substrate-premium-282462371.html (Accessed Feb 16, 2016)
Figure 1. Slow thiol diffusion coupled with a fast reaction leads to an advancing reaction interface.
Figure 2. Fast thiol diffusion coupled with a slow reaction leads to more uniform bond breakage.
Figure 3. Custom-designed SFTK cell
Figure 4. Output from a typical SFTK experiment










