Industry expert Tony O'Lenick explains the differences in structure and function between an emulsion and an invert emulsion...
Emulsions are systems composed of two or more immiscible materials, in which one material (the dispersed/internal phase) is suspended or dispersed throughout another material (the continuous/external phase) in separate droplets. The immiscible phases can be water, oil or silicone. When emulsions are made surfactants called emulsifiers are used to slow the process of separation of the immiscible phases. All emulsions are inherently unstable with the exception of spontaneously forming micro emulsions. All we can do is delay the day when the instability will arrive. Emulsions are classified by the continuous phase (external) and the discontinuous phase (internal). The use of homogenizers and other equipment to minimize droplet size will improve the stability of an emulsion.
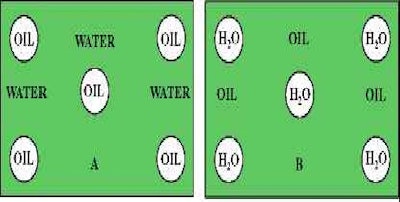
When naming the emulsion type, the first letter is the discontinuous phase. O/W is oil in water and is classified as an emulsion. W/O is water in oil and is classified as an invert emulsion. The graphic below shows the difference.
O/W Emulsion W/O Emulsion