Solubility
Solubility is a term used to describe a situation in which one material becomes clear and homogeneous in another. The two materials may be liquids, or they may be one liquid and one solid. Solubility relates to the balance between two opposing forces. The first is the force of attraction between the molecules of the material being dissolved to each other, and the second the force of attraction between the material being absorbed and the solvent. Simply put, if the force of attraction between the molecules of the solute is less than the force that is necessary to dissolve the material, the material dissolves.
To determine solubility, one must consider several factors, including temperature and concentration. Generally, a material is soluble at a concentration of 0.1M, as the material is clear at RT. Conversely, if a material is not clear at a concentration of 0.001M, it is insoluble.
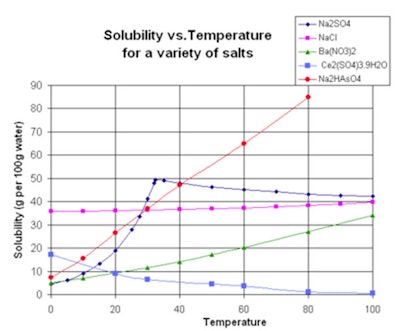
The solubility of a material increases as the temperature increases, but not always, as shown in Figure 1.1 While most materials follow this rule such as sodium hydrogen arsenate, some materials actually decrease in solubility as temperature increases. Gases are more soluble at low temperatures than at high temperatures.
The solubility of materials in different solvents is dependent upon many things and can vary based upon temperature, concentration and formulation additives. This can provide the formulator a way to deliver materials. If a material is delivered in an aqueous solution spread on the skin, water evaporating can change the deposition on the skin. The concept of solubility equilibrium will be discussed in another column, but it relates to a dynamic equilibrium between the concentration of soluble and insoluble compounds. This equilibrium can be altered by temperature or other formulation ingredients.
Partition Coefficient
Partition Coefficient is a concept that is a consequence of solubility and relates in that not all of a material will stay in an oil phase or a water phase. The partition coefficient results from most compounds being soluble to some extent in both organic and aqueous phases. The concentration of compound in the two phases is used to determine the partition coefficient.
The industry is familiar with using water/ether extraction in a separatory funnel, as illustrated in Figure 2.2 Knowing that some materials partition between hydrophilic (aqueous) and hydrophobic (oil) systems and understanding that this equilibrium is dynamic, allows for the formulation of products in which a material is delivered to the skin from over time from one phase to another. An example is delivery of a material to the skin as it becomes more oily.
The ability to partition compounds into different solvents is important to the cosmetic chemist. There is both oil and water in the skin, and the way actives are distributed into the phases will partly determine the effectiveness of the formulation in the active's delivery.
Reference
1. Solubility, Wikipedia, https://en.wikipedia.org/wiki/Solubility (accessed Apr 11, 2012)
2. Separatory Funnel, Harper College, www.harpercollege.edu/tm-ps/chm/100/dgodambe/thedisk/labtech/sepfun2.htm (accessed Apr 11, 2012)