The continuing debate over cosmetic preservatives has limited the number of accepted actives that can be practically used. As a result, a number of different methods and materials are used to boost the activity of the remaining acceptable preservatives. The boosting effects of chelating agents on preservatives are well-known. The study described here examines the influence of pH on chelating activity and boosting effects at both pH 7.0 and pH 5.0.
Preservative Preparation
Some of the most commonly used preservative systems for cosmetic products are blends of phenoxyethanol and parabens. Due to market demand, suppliers are putting great effort into developing alternatives to traditional preservative actives. By combining a well-known, accepted preservative active—phenoxyethanol—with an innovative, multifunctional cosmetic additive—ethylhexylglycerin—euxyl® PE 9010 is meeting the market requirement for new preservative concepts. euxyl® PE 9010 has been tested in more than 350 different cosmetic products, both alone and in comparison studies vs. different traditional cosmetic preservative systems. These tests have proven euxyl® PE 9010 to have similar efficacy to traditional phenoxyethanol/paraben blends.
Chelating agents have been widely used to boost the activity of cosmetic preservative systems. The efficacy in this regard of tetrasodium EDTA and tetrasodium dicarboxymethyl glutamate has been discussed in previous publications. As pH has limited influence on the efficacy of the preservative blend euxyl® PE 9010, this study attempts to isolate the influence of pH on the chelating agent used.
Test Method
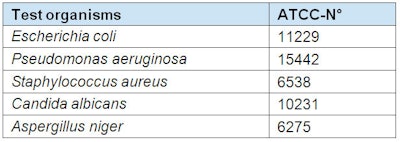
Dilutions of test preservative and combinations of preservative/chelating agent were prepared using sterile hard water according to the European standard for testing chemical disinfectants and antiseptics (see Water Dilution According to European Standard). Sample 50-ml portions of the end solutions were each inoculated with 0.5 ml microorganism suspension (initial microorganism count ~108 cfu/ml) and stirred. Table 1 shows the test organisms used.
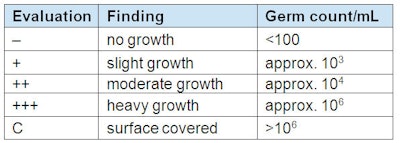
These solutions were streaked out onto tryptone soya agar or sabouraud-dextrose 4% agar after 1, 3, 6, and 24 hr. The cultures were incubated for 48 hr at 37°C, except for Aspergillus niger, which was incubated for 72 hr at 25–27°C. Evaluations were made on the basis of semi-quantitative assessments of the microbial growth of the streaks (see Table 2).
Test Concentrations and Chelating Agents
To obtain a clear differentiation between the preservative with and without chelating agents, the lowest effective concentration of preservatives was used. For euxyl® PE 9010, this amount is 0.75%.
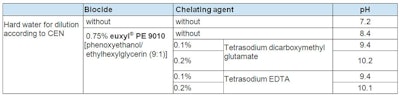
The chelating agents used are shown in Table 3. These agents are dosed at 0.1% and 0.2%, and calculated as active matter. Due to the alkalinity of the chelating agents, the final solutions were adjusted to pH 7.0 and pH 5.0, respectively. Additionally, the influence of the acid used for neutralization was examined. Hydrochloric acid (HCl) was chosen as inorganic acid and citric acid as organic alpha-hydroxyl acid since the formed citrates can support the chelating effect. Table 4 shows the pH values before adjustments.
Results
Figures 1-12 show ingredient combination variations and the resulting boosting effects of the chelating agents at pH level of 5.0 and 7.0 on euxyl® PE 9010. As a control, Figure 1, at pH 7.0, and Figure 2, at pH 5.0, show results without preservatives. Figure 3 shows the results of 0.75% euxyl® PE 9010 without a chelating agent at a pH of 7.0, while Figure 4 shows the same but at a pH of 5.0. In Figure 5, 0.1% tetrasodium EDTA has been added to the 0.75% euxyl® PE 9010 at a pH of 7.0 and again, Figure 6 shows the same but at a pH of 5.0.
Figure 7 shows results when the amount of tetrasodium EDTA is increased to 0.2% at a pH of 7.0 while maintaining the other variables, and Figure 8, again, shows the same but at a pH of 5.0. Figure 9 shows results, at a pH 7.0, of 0.75% euxyl® PE 9010 with 0.10% tetrasodium dicarboxymethyl glutamate in place of the tetrasodium EDTA; and Figure 10, the same, but at a pH 5.0. Finally, Figure 11 shows the results of the same combination of materials at a pH of 7.0, but with an increase in the tetrasodium dicarboxymethyl glutamate to 0.20%; and Figure 12 shows the same at a pH of 5.0
Overall, the chelating agents were found to exhibit a better boosting effect at pH 5.0 for E. coli and Ps. aeruginosa. For Staph. aureus, especially with tetrasodium dicarboxymethyl glutamate, the result at pH 7.0 was better. Finally, the efficacy for Candida albicans was significantly worse at pH 5.0.
Chelating agents have long been known to boost the efficacy of cosmetic preservative systems. This study highlights the importance of choosing the appropriate pH and pH adjuster based on the microbiological susceptibility of the finished formula.
Disclaimer:
The above paid-for content was produced by and posted on behalf of the Sponsor. Content provided is generated solely by the Sponsor or its affiliates, and it is the Sponsor’s responsibility for the accuracy, completeness and validity of all information included. Cosmetics & Toiletries takes steps to ensure that you will not confuse sponsored content with content produced by Cosmetics & Toiletries and governed by its editorial policy.