
The concepts of hydrophilic-lipophilic balance (HLB) and phase inversion temperature (PIT) are both helpful for making emulsions. However, the HLB is a system used help to select PEG-containing emulsifiers by considering their composition—specifically, the percentage of PEG in the molecule.
In contrast, PIT refers to the temperature during processing at which emulsions have improved stability.1 Here, Tony O’Lenick demonstrates the benefits of each, with added contributions from Kelly Dobos, Ken Klein and Thomas O’Lenick, Ph.D.
HLB Calculation and Application
As Kelly Dobos explains,2 “William C. Griffin developed a way to streamline the selection of surfactants by utilizing the ratio of the hydrophobic to the hydrophilic portion of the molecule. This method is referred to as the Hydrophile-Lipophile Balance (HLB) method. Griffin first presented this method3 at meeting of the Chicago Chapter of the Society of Cosmetic Chemists in 1949, and it is still widely used today.
“For example, a typical nonionic emulsifier, e.g., laureth-4, contains ethylene oxide groups or polyhydric alcohol hydrophilic portions with a fatty alcohol hydrophobic portion. The HLB for a nonionic surfactant can be calculated as: HLB = Weight % Hydrophile/5. Therefore, the HLB calculation for laureth-4 is determined as follows.
Molecular weight of ethoxylated portion = 176
Molecular weight of lauryl alcohol = 186
Wt.% Hydrophile = (176/(176+186)) x 100 = 48.6%
Thus, HLB = 48.6/5 = 9.7
“Based on the calculation, surfactants with high HLB values will be more water-soluble and those with low HLB values are more oil-soluble; note the division by five simply allows for a compact, easy-to-use scale. While this calculation is simple, most surfactant HLB values are readily available through literature references and surfactant suppliers, and will not need to be calculated.”
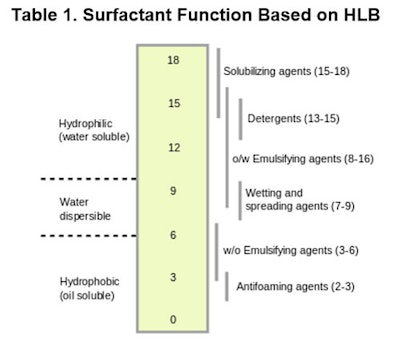
Additionally, the HLB value allows one to predict the function of a surfactant, as shown in Table 1.
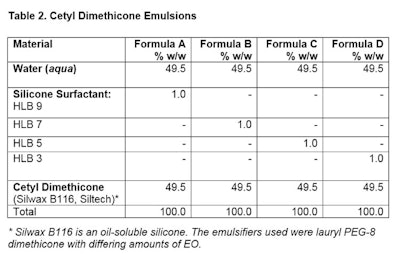 Formula procedure: Place emulsifier into oil phase. Mix well, noting clarity. Heat both phases to 50°C. Add water phase to oil phase and using mixer, mix for 120 sec. Note appearance after 3 hr and 24 hr.
Formula procedure: Place emulsifier into oil phase. Mix well, noting clarity. Heat both phases to 50°C. Add water phase to oil phase and using mixer, mix for 120 sec. Note appearance after 3 hr and 24 hr.Effect of HLB Change
To accelerate formula separation and speed up the evaluation, a handheld mixer was used (see Figure 1). This was not intended for commercial production, but simply to reduce the time necessary to get results.
 Mixing was conducted for 5 min at high speed. Figure 2 shows the emulsions after 3 hr at RT; Figure 3 shows the same emulsions after 24 hr.
Mixing was conducted for 5 min at high speed. Figure 2 shows the emulsions after 3 hr at RT; Figure 3 shows the same emulsions after 24 hr.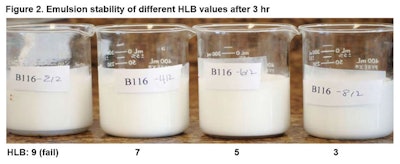 Clearly, the HLB will have a profound effect on making the emulsion, and is matched to the oil chosen. Here, the process was the same; the HLB was used to determine the selection of the proper emulsifier.
Clearly, the HLB will have a profound effect on making the emulsion, and is matched to the oil chosen. Here, the process was the same; the HLB was used to determine the selection of the proper emulsifier.PIT and Emulsion Selection
One of the best methods available for choosing emulsifiers is the Phase Inversion Temperature (PIT), a system developed by Shinoda3, 4 based on the temperature above which an ethoxylated surfactant becomes insoluble in water.
Ken Klein5 explained it as follows: “Why are ethoxylated fatty materials soluble in water? The answer is the hydrogen bonding between the epoxide (as it was once) oxygen of ethylene oxide, and the hydrogen of water. Many have observed the reverse cloud point seen with ethoxylated materials.
"It is well-known that as temperature is increased, ethoxylated surfactants become less water-soluble. This somewhat surprising observation is easily explained by considering that, as the temperature increases, the molecules exhibit more movement/vibration. Thus, hydrogen bonding is inhibited, the ethoxylate loses its water solubility, and cloudiness results.”
One additional point is that above the reverse cloud point, the surfactant becomes more stable in the oil phase than in the water phase. Upon cooling, the partitioning of the surfactant into the water phase increases, resulting in a more stable emulsion process. PIT, cloud point, inverse cloud point and reverse cloud point are all terms used to refer to the loss of solubility as a non-ionic is heated. Polymer chemist Thomas O’Lenick, Ph.D., explains, in the next section.
PIT Evaluation
To put the PIT approach to the test, isolaureth-6a was used. This surfactant was chosen since it has a low high cloud point and the proper approximate HLB value. It would therefore allow the study of the effect of the PIT process on emulsion properties using the same HLB.
Figure 4 shows the clarity of a 1% solution of isolaureth-6 in water at 20°C on the left, and the same 1% solution of isolaureth-6 in water at 36°C on the right.
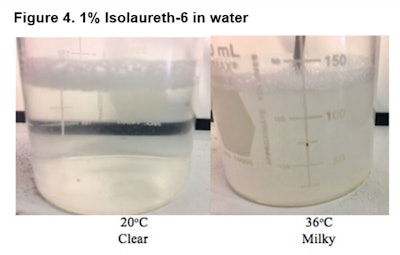 The solution in the image above the high cloud point has become turbid and will separate over time. Figure 5 shows that a 1% solution of isolaureth-6 in olive oil is soluble and clear at both temperatures.
The solution in the image above the high cloud point has become turbid and will separate over time. Figure 5 shows that a 1% solution of isolaureth-6 in olive oil is soluble and clear at both temperatures. Since the emulsifier is insoluble in water above 36°C, and is still soluble in the oil at 36°C and above, the surfactant partitions more into the oil phase and less in the water phase. This is the key as to why PIT surfactants work.
Since the emulsifier is insoluble in water above 36°C, and is still soluble in the oil at 36°C and above, the surfactant partitions more into the oil phase and less in the water phase. This is the key as to why PIT surfactants work.A simple formulation, again mixed with a handheld mixer, was chosen for a rapid screening method as a way to evaluate the effects of both a cold temperature and PIT temperature process. The formulation for both processes were the same and shown in Table 3.
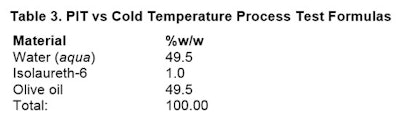 Cold temperature procedure: Place emulsifier into oil phase. Mix well, noting clarity. Do not heat either phase. Add water phase to oil phase and using mixer, mix for 120 sec. Note appearance after 3 hr and 24 hr.
Cold temperature procedure: Place emulsifier into oil phase. Mix well, noting clarity. Do not heat either phase. Add water phase to oil phase and using mixer, mix for 120 sec. Note appearance after 3 hr and 24 hr.PIT temperature procedure: Place emulsifier into oil phase. Mix well, noting clarity. Heat both phases to 50°C. Add water phase to oil phase and using mixer; mix for 120 sec. Note appearance after 3 hr and 24 hr.
Initial results: At the end of the process, both emulsions were yellowish liquids. There was no noticeable difference between them. They were re-evaluated in 24 hr.
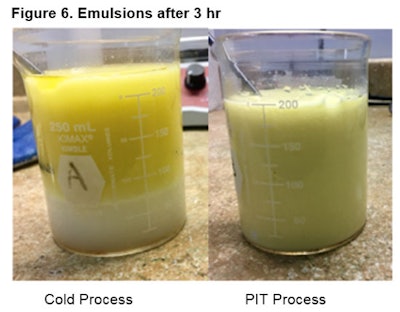
At 3 hr: Figure 6 shows the emulsions after 3 hr at RT; clearly, the PIT fared better, with no apparent separation.
 After 24 hr: Figure 7 shows the emulsions 24 hr later.
After 24 hr: Figure 7 shows the emulsions 24 hr later.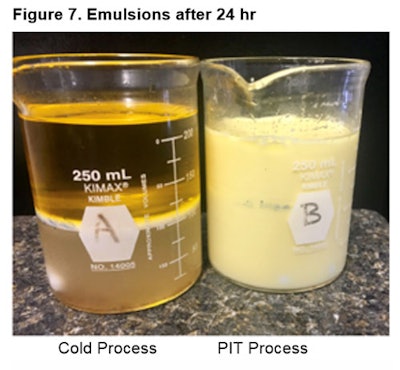 After 24 hr, clearly there was a big difference in emulsion quality when comparing the two processes as shown in Figure 4. The emulsifier was identical, i.e., chosen by selecting an emulsifier with a “close HLB” and a “low high cloud point.” The HLB for this emulsion therefore needs to be adjusted further using a lower HLB.
After 24 hr, clearly there was a big difference in emulsion quality when comparing the two processes as shown in Figure 4. The emulsifier was identical, i.e., chosen by selecting an emulsifier with a “close HLB” and a “low high cloud point.” The HLB for this emulsion therefore needs to be adjusted further using a lower HLB.Conclusion
HLB and PIT are both important for making emulsions. They provide different information, however, so both should be used to optimize emulsions.
a Tergitol TMN-6 (INCI: Isolaureth-6), CAS No. of 60828-78-6, is a registered trademark of Dow Chemical.6










