Epigenetics describes a new paradigm in genetics whereby gene expression is modulated without altering the genetic code contained in the DNA sequence.1 The regulation of gene expression can be affected through at least three different mechanisms (see Figure 1). The first is via DNA methylation, where cytosine molecules are methylated by a DNA methyltransferase enzyme. This modification of DNA sequences typically leads to gene silencing, which will have downstream biological effects.
Second, histone proteins with which DNA is wrapped can be posttranslationally modified with methyl groups and acetyl groups, leading to increases and decreases in gene activity. Of these modifications, acetylation has been shown to have the most well-characterized effect on the transcription and subsequent translation of genes.
Finally, microRNAs also can function epigenetically by intercepting and annealing to messenger RNA (mRNA) molecules transcribed from genes, culminating in the sequestration or degradation of the mRNA and preventing protein synthesis at the ribosome. Both DNA methylation and microRNA-induced posttranscriptional gene silencing have been extensively reviewed elsewhere,2, 3 and as such will not be the focus of this review. Here, the modulation of histone and nonhistone protein acetylation will be the focus, as well as how this epigenetic modification can be translated into next-generation cosmetic applications.
Mechanisms of Posttranslational Modification
Histone and nonhistone proteins are potential substrates for a variety of posttranslational modifications, including but not limited to: acetylation, methylation, phosphorylation, ubiquitination, sumoylation (small ubiquitin-like modifier, adenosine diphosphate (ADP) ribosylation, deamination and proline isomerization.4
Modulation of the acetylation state of histones and nonhistone proteins occurs by two different groups of enzymes. The transfer of acetyl groups to the lysine residues is catalyzed by histone acetyl transferases (HATs); the flip side is the removal of acetyl modifications by histone deacetylases (HDACs). This dynamic on/off mechanism is often referred to as the HAT-HDAC switch.
Modern science has developed a group of chemical compounds referred to as HDAC inhibitors (HDACis) that, as the name suggests, can inhibit the function of HDACs, allowing for the acetylation state to remain “on” and for increased transcriptional activity to persist.
The acetylation and deacetylation of histone and nonhistone proteins involves the attachment of an acetyl group to lysine residues protruding from these proteins.5 In the context of histones, this modification decreases the overall positive charge of the protein, thereby weakening its electrostatic interaction with the negatively charged phosphate groups on the genomic DNA. This loosens the DNA wrapped around the histone proteins, allowing for chromatin remodeling factors and transcription factors to interact with the DNA and promote gene expression. Regions of genomic DNA that exhibit increased gene expression or transcriptional activity are described as euchromatin, while the contrasting condition of condensed, inactive DNA is termed heterochromatin.
The case of acetylation and deacetylation of nonhistone proteins, the so-called acetylome, also has received a significant amount of attention. The acetylation of nonhistone proteins can modulate their DNA-binding ability, protein stability, ability to interact with other proteins, as well as their capacity to induce transcriptional activation. Intriguingly, the majority of proteins that are subject to reversible acetylation are involved in cellular signaling related to oncogenic and immune pathways. Indeed, the first nonhistone protein identified as a substrate for HATs was the well-known tumor suppressor protein p53.5 Currently, many reports of HDACis ameliorating a variety of conditions have emerged. This review will attempt to coalesce and refine them into a cogent discussion, with an emphasis on their utility for treating several skin conditions.
HDACis allow for the acetylation state to remain 'on' and for increased transcriptional activity to persist.
HATs and HDACs
As stated, one of the ways gene expression is naturally modulated by cells is through the posttranslational modification of histone proteins by a group of antagonistic enzymes: HATs and their counterparts, HDACs. Importantly, contrary to their names, they also affect nonhistone proteins. Generally speaking, acetylation of histones by HATs produces increased gene expression, while deacetylation results in diminished expression. Moreover, HDACs have been implicated in a variety of different cancers, including but not limited to: cutaneous T-cell lymphoma, myeloid leukemia, breast cancer, lung cancer, renal cancer, head and neck cancer, prostate cancer and colorectal cancer.
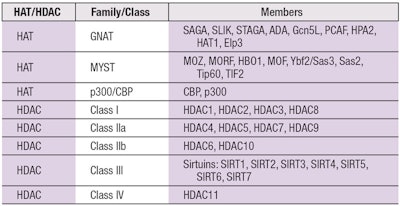
HATs: There are no less than 30 different HATs spread across multiple protein families, which include the GNAT family, the MYST family and the CBP/p300 family (see Table 1). There also are protein factors that function as HATs but do not conform to these three families.
The HATs belonging to the GNAT family, standing for “General Control Nonderepressible” (GCN5) related N-acetyltransferases, include acetylate histones H2B, H3, and H4 as well as some nonhistone proteins. The members of the MYST family include MOZ, YBF2, SAS2 and TIP60, which is where the family gets its name. Finally, the p300 and CREB-binding protein (CBP) family are known adaptor molecules that not only relax chromatin structure through their HAT activity, but also recruit important transcription factors to the site of gene expression including RNA polymerase II.
The global epigenetics market is projected to reach USD $1.60 billion by 2022, growing at a CAGR of 13.3% from 2017 to 2022.
-MarketsandMarkets
HDACs: To date, there are four known classes of HDACs, of which there are 18 members grouped according to differences in their respective amino acid sequences as well as whether their respective active sites require a bound zinc (Zn) ion or nicotinamide adenine dinucleotide (NAD+) (see Table 1).
HDAC classes with Zn bound in the catalytic domain include Class I, II and IV, while Class III has a bound NAD+. Class I comprises HDAC1, HDAC2 and HDAC3. Class II is subdivided into IIa with HDAC4, HDAC5, HDAC7 and HDAC9 and IIb with HDAC6 and HDAC10. Lastly, there is a singular Class IV (HDAC11) that is reportedly unique with respect to the other HDACs in that it remains largely localized to the nucleus and does not appear to participate in any of the known supramolecular HDAC complexes.6
Class III HDACs, aka sirtuins: Much interest has recently focused on Class III HDACs, also known as sirtuins (SIRT1, SIRT2, SIRT3, SIRT4, SIRT5, SIRT6 and SIRT7), due to their involvement in pathways regulated by resveratrol, a compound believed to extend the human lifespan. Beautifully summarized in a 2008 New York Times article titled, “New Hints Seen That Red Wine May Slow Aging,” resveratrol is a molecule isolated from certain red wines that has been speculated to be responsible for burning off calories in a manner mimicking exercise. This has already led to the development of resveratrol pills, in hopes of stimulating a person’s metabolism in the absence of actual physical activity.
Interestingly, resveratrol also has been reported to inhibit Class I, II and IV HDACs.4, 7 Note that not all Class III HDACs are limited to deacetylase activity. SIRT4 and SIRT6 can function as ADP-ribosyl transferases. SIRT5 can function as a de-malonylase and de-succinylase. SIRT6 also exhibits demyristoylase and depalmitoylase activity.
HDAC Inhibitors
As mentioned, HDACis that also function as nonhistone deacetylase inhibitors represent a group of compounds that abrogate the effects of HDACs, culminating in less-condensed chromatin that allows for increased gene transcription and alters the functionality of nonhistone protein targets.8 Most HDACi molecules interact with HDACs via a Zn ion that HDACs need for catalyzing the removal of acetyl groups.9, 10 The remaining HDACis attack the NAD+ in the catalytic sites of the sirtuin class of HDACs.
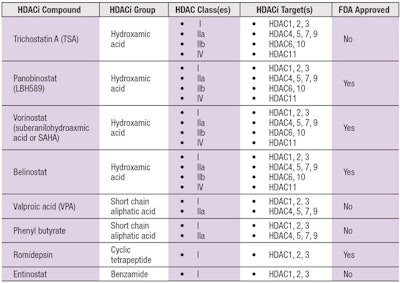
The HDACis that target Zn-containing catalytic region Class I, IIa, IIb and IV HDACs can be categorized into one of five different classes: hydroxamic acids, cyclic tetrapeptides, benzamides, electrophilic ketones and aliphatic acids. The currently known HDACi compounds are summarized in Table 2, which includes both synthetic and naturally occurring molecules.
Of the four U.S. Food and Drug Administration (FDA)-approved HDACis, three are hydroxamic acid derivatives: vorinostat, panobinostat and belinostat; and one is a cyclic tetrapeptide: romidepsin.6 More than 15 different HDACi compounds have been entered into more than 350 clinical trials.11
Depending on a particular HDACi, its impact can be specific to an individual HDAC or it can have broad effects on multiple HDACs. For example, the fungal protein Trichostatin A (TSA) is classified as a pan-HDACi given that its inhibitory effects span Class I, IIa, IIb and IV HDACs. In contrast, the HDACi activity of Entinostat is limited to Class I HDACs; thus, it is classified as a selective HDACi.
The effects that HDACis elicit impact a variety of cellular processes, including but not limited to: autophagy, metabolism, inflammation, fibrogenesis, cell cycle arrest and immune responses. Therefore, there is significant merit to the investigation of these compounds as therapeutics for a variety of health concerns and potential applications.
For example, HDAC inhibitors such as TSA have received considerable attention recently as pharmaceutical companies are capitalizing on their utility as anti-cancer agents.9, 12-14 Moreover, they have been explored in other contexts to combat parasitic infections, modify people’s moods, and even purge latent human immunodeficiency virus (HIV) infections.15-17 Interestingly, at much reduced concentrations, several HDACis show potent anti-inflammatory activity6, 16, 18-21 and this latter functionality has caught the attention of the skin care industry.
Skin-related Benefits of Histone Acetylation
Of the genes whose expressions are augmented by the inhibition of histone deacetylation, one is of particular utility to skin health: the specific inhibition of HDAC3 promotes the expression of aquaporin-3 (AQP3) (see Figure 2). AQP3 is a protein responsible for the transport of water and glycerin into and out of cells.
The application of pan HDACi’s to skin cells has been found to trigger the upregulation of AQP3 expression in as short a period as 24 hr. The glycerin cargo transported via AQP3 has been shown to promote wound healing, skin hydration and skin elasticity. Furthermore, scientists are now considering that increased expression of AQP3 through the inhibition of HDAC3 via HDACis could be a therapeutic approach for psoriasis, vitiligo and atopic dermatitis.22
Class III HDACis, also known as sirtuins, are involved in pathways regulated by resveratrol.
Anti-inflammatory Effects
Beyond their utility against cancer, HDACis are highly effective anti-inflammatory agents. Several reports have demonstrated that various skin conditions arise from pro-inflammatory responses that, in some cases, are due HDAC activity. As such, the anti-inflammatory impact HDACis can promulgate has been investigated as an effective therapeutic tool to combat these skin diseases.23, 24 Additionally, HDACis reportedly affect the pathogenesis of certain viruses, which could be expanded to include those that infect the skin.
Anti-fibrotic Effects
HDACis have also been explored for their ability to impact tissue fibrosis; one article reviewed HDACi treatments that produced anti-fibrotic effects for a variety of disorders including but not limited to renal fibrosis, cystic fibrosis, systemic sclerosis, cardiac fibrosis and pulmonary fibrosis.25 The utility of anti-fibrotic HDACis certainly could be applied to wound healing and keloid fibroblasts. In fact, TSA has already been investigated for its ability to counteract keloid formation, where its application successfully decreased collagen synthesis and catalyzed the apoptotic response in keloidal fibroblasts.26
Anti-melanogenic Effects
A final potential use of HDACis worth mentioning is skin brightening; a scientific publication from 2008 describes one HDACi downmodulating the expression of the microphthalmia-associated transcription factor (MITF), which is immediately upstream from tyrosinase (TYR) in the gene pathway for melanogenesis.27
MITF is also required for the normal development of melanocytes and its dysregulation can lead to melanoma formation. In the study described, four different HDACis were tested for their ability to regulate pigmentation: sodium butyrate, TSA, SAHA and LBH589. The topical introduction of each these, individually, to the skin of mice was shown to reduce skin pigmentation.
Practical Application
Currently, several commercial endeavors are under way to harness the potential of these epigenetic applications. One aims to use information gleaned from an individual’s epigenome to provide personalized skin care products. Others include efforts to develop and market a line of vegan anti-aging products, and a line of creams to reprogram skin cells and boost the production of collagen, elastin and hyaluronic acid. Moreover, a cursory survey of existing patent applications shows that many institutions are harnessing HDACis for applications ranging from combatting wrinkle formation, to ameliorating rosacea.
One focus of the authors’ company is the investigation of naturally occurring compounds that can function as HDAC inhibitors for downstream restorative properties. Specifically, a bio-active material containing extracts from the plant Agrimonia eupatoria, more commonly known as agrimonya, was found to downregulate the production of several inflammatory signals in the skin, including: interferon gamma-induced protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP-1), macrophage colony-stimulating factor (M-CSF) and macrophage inflammatory protein 1 beta (MIP-1β). Furthermore, a separate, variant formulation produced from the extract has shown anti-fibrotic activity, with clear applications for wound healing. Small doses of the material also demonstrate a reduction in collagen production; and most striking, this distinct formulation also behaves as an HDACi, as evidenced in in vitro assays where potential HDACi’s can be screened for their impact on HDAC activity and compared with a well-defined HDACi (data not shown).
Conclusions
As can be gleaned from this overview of compounds exhibiting HDAC inhibitor activity, these materials represent attractive options for restorative skin care treatments. HDAC inhibitors have already shown substantial utility as anti-cancer compounds at much higher doses and have been approved by the FDA for human use. Thus, these molecules and their positive impact on skin care maladies already benefit from a validated safety profile at substantially higher doses and, from the information described herein, are certainly worth greater study.
Beneficial effects elicited from HDACi treatments such as pro-hydration, pro-elasticity, anti-fibrosis and anti-inflammation are rapidly putting these compounds in the spotlight of the cosmetic care industry. Many more companies beyond those specifically mentioned are endeavoring to create HDACi-based remedies, which will be marketed in the coming years.
References
- Goldberg, A. D., Allis, C. D., and Bernstein, E. (2007). Epigenetics: a landscape takes shape. Cell 128, 635-638.
- Lawrence, P., Ceccoli, J. (2017). Advances in the Application and Impact of MicroRNAs as Therapies for Skin Disease. BioDrugs 31, 423-438.
- Li, Y., Sawalha, A. H., Lu, Q. 2009. Aberrant DNA methylation in skin diseases. J Dermatol Sci 54, 143-149.
- Bassett, S. A., Barnett, M. P. (2014). The role of dietary histone deacetylases (HDACs) inhibitors in health and disease. Nutrients 6, 4273-4301.
- Glozak, M. A., Sengupta, N., Zhang, X., Seto, E. (2005). Acetylation and deacetylation of non-histone proteins. Gene 363, 15-23.
- Hull, E. E., Montgomery, M. R., Leyva, K. J. (2016). HDAC Inhibitors as Epigenetic Regulators of the Immune System: Impacts on Cancer Therapy and Inflammatory Diseases. Biomed Res Int 2016, 8797206.
- Venturelli, S., et al. (2013). Resveratrol as a pan-HDAC inhibitor alters the acetylation status of histone [corrected] proteins in human-derived hepatoblastoma cells. PLoS One 8, e73097.
- Seidel, C., Schnekenburger, M., Dicato, M., Diederich, M. (2012). Histone deacetylase modulators provided by Mother Nature. Genes Nutr 7, 357-367.
- Lane, A. A., Chabner, B. A. (2009). Histone deacetylase inhibitors in cancer therapy. J Clin Oncol 27, 5459-5468.
- Xu, W. S., Parmigiani, R. B., Marks, P. A. (2007). Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene 26, 5541-5552.
- Zwergel, C., Stazi, G., Valente, S., Mai, A. (2016). Histone Deacetylase Inhibitors: Updated Studies in Various Epigenetic-Related Diseases. J Clin Epigenet 2, 1-15.
- Meidhof, S., et al. 2015. ZEB1-associated drug resistance in cancer cells is reversed by the class I HDAC inhibitor mocetinostat. EMBO Mol Med 7, 831-847.
- Meng, J., et al. (2014). The anti-tumor histone deacetylase inhibitor SAHA and the natural flavonoid curcumin exhibit synergistic neuroprotection against amyloid-beta toxicity. PLoS One 9, e85570.
- Mottamal, M., Zheng, S., Huang, T. L., Wang, G. (2015). Histone deacetylase inhibitors in clinical studies as templates for new anticancer agents. Molecules 20, 3898-3941.
- Schroeder, F. A., Lin, C. L., Crusio, W. E., Akbarian, S. (2007). Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol Psychiatry 62, 55-64.
- Dinarello, C. A., Fossati, G., Mascagni, P. (2011). Histone deacetylase inhibitors for treating a spectrum of diseases not related to cancer. Mol Med 17, 333-352.
- Matalon, S., Rasmussen, T. A., Dinarello, C. A. (2011). Histone deacetylase inhibitors for purging HIV-1 from the latent reservoir. Mol Med 17, 466-472.
- Cantley, M. D., Fairlie, D. P., Bartold, P. M., Marino, V., Gupta, P. K., Haynes, D. R. (2015). Inhibiting histone deacetylase 1 suppresses both inflammation and bone loss in arthritis. Rheumatology (Oxford) 54, 1713-1723.
- Leoni, F., et al. (2005). The histone deacetylase inhibitor ITF2357 reduces production of pro-inflammatory cytokines in vitro and systemic inflammation in vivo. Mol Med 11, 1-15.
- Lewis, E. C., et al. (2011). The oral histone deacetylase inhibitor ITF2357 reduces cytokines and protects islet beta cells in vivo and in vitro. Mol Med 17, 369-377.
- Lin, H.S., et al. (2007). Anti-rheumatic activities of histone deacetylase (HDAC) inhibitors in vivo in collagen-induced arthritis in rodents. Br J Pharmacol 150, 862-872.
- Choudhary, V., et al. (2017). Regulation of the Glycerol Transporter, Aquaporin-3, by Histone Deacetylase-3 and p53 in Keratinocytes. J Invest Dermatol 137, 1935-1944.
- Shi, Y.L., et al. (2012). Histone deacetylases inhibitor Trichostatin A ameliorates DNFB-induced allergic contact dermatitis and reduces epidermal Langerhans cells in mice. J Dermatol Sci 68, 99-107.
- Kim, Y., et al. (2012). Histone deacetylase 3 mediates allergic skin inflammation by regulating expression of MCP1 protein. J Biol Chem 287, 25844-25859.
- Pang, M., Zhuang, S. (2010). Histone deacetylase: a potential therapeutic target for fibrotic disorders. J Pharmacol Exp Ther 335, 266-272.
- Diao, J. S., et al. (2011). Trichostatin A inhibits collagen synthesis and induces apoptosis in keloid fibroblasts. Arch Dermatol Res 303, 573-580.
- Yokoyama, S., et al. (2008). Pharmacologic suppression of MITF expression via HDAC inhibitors in the melanocyte lineage. Pigment Cell Melanoma Res 21, 457-463.