Irritant contact dermatitis (ICD) is a common form of irritation that can occur when human skin is exposed to potential irritants such as surfactants, cutting oils, etc.1, 2 Minimizing exposure to such irritants is recommended but often not practical since exposure is unavoidable in some occupations including farming, firefighting, medicine, etc. To counteract or suppress irritation responses in the skin, anti-irritant agents are employed. These ingredients, whether naturally occurring or man-made, used alone or in formulations, possess the capacity to reduce irritation caused by acute and chronic exposure to irritants.3
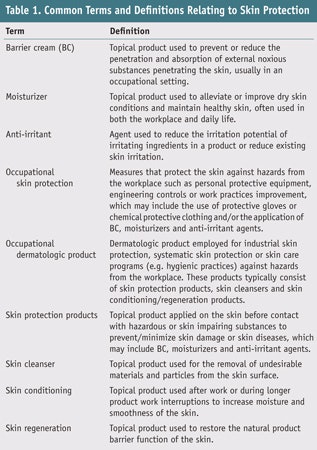
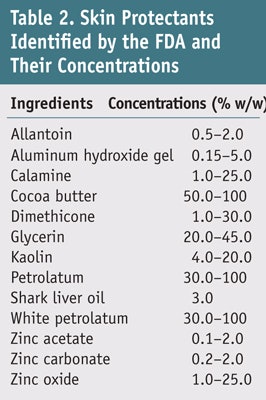
To fully prevent or reduce the risk of developing ICD, anti-irritant agents such as barrier creams (BCs) and moisturizers are widely utilized.4–9 Though BCs and moisturizers are not identical, probably due to their ambiguous definitions, the terms BC and moisturizer are often used interchangeably in the literature and the marketplace. The target of BCs is to prevent external noxious substances from penetrating skin, usually in an occupational setting,5, 7–9 whereas moisturizers are frequently used for dry skin conditions as well as to maintain healthy skin.4, 6, 9–11 However, moisturizers and BCs share characteristics, thus it can be difficult to distinguish between the two. Therefore it is suggested that the standard term skin protectant be used when referring to anti-irritants;12 several terms and definitions commonly used in reference to skin protection are summarized in Table 1. Although numerous ingredients have been formulated into finished skin protection products, the US Food and Drug Administration (FDA) has only endorsed 13 ingredients for over-the-counter (OTC) products (see Table 2).
Based on an extensive literature review, this column describes studies and data related to the proposed efficacy of anti-irritant agents for reducing ICD in human skin. To compile this review, a literature search in PubMed, EMBASE and Scopus was conducted, and only research discussing either the prevention or treatment of irritation in human skin was considered. Studies and data conducted on non-human skin were excluded, and emphasis was placed on the studies that included quantitative and qualitative results as well as those that followed evidence-based dermatological guidelines.
For the purpose of this review, anti-irritant is defined as a moiety that either inhibits (prevents) or treats ICD. Specific focus was placed on the clinical markers of irritation—i.e., edema, erythema, vesiculation and diminished barrier function—as these are more readily and objectively assessed with visual scoring criteria and bioengineering measurements.
Irritant Reaction
As noted, ICD is the result of an unspecific amount of damage to the skin from contact with irritating chemical substances.1, 4 Exposure to irritants such as solvents, detergents and even water13 can lead to stratum corneum damage, resulting in skin barrier impairment.4 While the exact mechanisms of irritant reaction are not completely understood, it seems likely that there is an immunologiclike component to the irritant response.1 The clinical appearance of ICD varies depending on multiple internal and external factors.1, 3 Airborne ICD may develop in uncovered skin areas, mostly in the face and neck, after exposure to volatile irritants or vapor. Prophylactic measures can be taken to reduce the risk of ICD—and oftentimes, BCs and moisturizers as well as anti-irritant agents may play a key role in this strategy.
Barrier Cream Efficacy
Protective gel: Lupulescu and Birmingham observed the ultrastructural and relief changes of human epidermis either left unprotected or treated with a protective gel following exposure to acetone and kerosene. Unprotected skin showed cell damage and a disorganized pattern in the upper layers of epidermis. However, skin treated with the protective agent showed substantially reduced ultrastructural and relief changes of epidermis cells when exposed to solvent.
Bioengineering evaluation of BCs: Grunewald et al. evaluated BC protective effects using bioengineering techniques and a sodium lauryl sulfate (SLS) repetitive washing model on 15 human volunteers. All BCs reduced the deterioration of skin functions after one week of repetitive washing. Subsequently, researchers also found that urea and glycerol o/w emulsions provided greater protection than three commercial BCs when tested against the lipophilic irritant toluene after seven days of repetitive irritation.
The effectiveness of BCs was measured on human subjects against dye indicator solutions including methylene blue in water and oil red O in ethanol, where methylene blue was representative of model hydrophilic compounds and oil red O was representative of lipophilic compounds. Each solution was applied at 5% to untreated and BC-pretreated skin with the aid of aluminum occlusive chambers for 0 hr and 4 hr. Post application time, materials were removed and consecutive skin surface biopsies (SSBs) were obtained. The amount of dye penetrating into each strip was determined colorimetrically. Two model creams exhibited protective efficacy but one enhanced the cumulated amount of dye—i.e., it failed to exhibit protective effect against dye indicators.
Four BCs vs. four irritants: Schlüter-Wigger and Elsner compared four commercially available BCs with four standard irritants: 10% SLS, 1% sodium hydroxide (NaOH), 30% lactic acid (LA) and undiluted toluene in a repetitive irritation test (RIT) on humans for 12 days. Irritation was assessed by visual scoring, transepidermal water loss (TEWL) measurements and colorimetry. All products were effective against SLS irritation. Nevertheless, no BC provided significant protection against toluene. Three of the four products tested showed a partially protective effect against all ionic irritants, while the fourth showed less protection against SLS and NaOH, and even amplification of inflammation by toluene.
Petrolatum skin protection: Wigger-Alberti and Elsner evaluated the protective effects of petrolatum utilizing the same model and noted that petrolatum was effective against SLS, NaOH and LA irritation; it also provided moderate protection against toluene. Subsequently, the researchers examined the efficacy of three other BCs and petrolatum against 10% SLS, 0.5% NaOH, 15% LA and undiluted toluene in an RIT on human volunteers for nine days. All BCs exhibited a significant protective effect against irritation by SLS, NaOH and LA, although less efficacy was observed against toluene. In another 12-day RIT study, white petrolatum provided a significant protective effect against SLS, NaOH, and TOL but with less protective effect against LA irritation.
BC protection against water: De Fine Olivarius et al. determined the efficacy of various BC and moisturizers in protecting skin against water. An aqueous solution of crystal violet was applied to the skin after pretreatment with different formulas and its color intensity was evaluated. The BC with particles gave the best immediate protection (dorsal 76%, volar 69%). The moisturizer was intermediately protective (dorsal 57%, volar 34%), while little protection was found for the silicone-containing cream (dorsal 16%, volar 10%).
Lipophilic BC protection from acute ICD: Fartasch et al. investigated the protective capacity of a lipophilic BC on acute ICD by TEWL measurement. Application of the BC before and during irritation showed a decrease TEWL enhancement with 0.5% SLS by 58% (back) and 49% (arm), and after irritation with 0.75% SLS by 56% (back) and 43% (arm).
PFPE irritant protection: Elsner et al. evaluated o/w emulsions containing 0.5%, 1.0%, 2.0% and 4.0% perfluoropolyethers (PFPE) against four irritants: 10% SLS, 0.5% NaOH, 15% LA and undiluted toluene in an RIT on the human back. Irritation was assessed by visual scoring, TEWL and colorimetry. All PFPE preparations significantly suppressed irritation caused by SLS and NaOH. However, only the 4% PFPE preparation was significant against LA and toluene.
In vivo protectant screening: Zhai et al. introduced an approach for screening protectants in vivo in human subjects. Two acute irritants, SLS and the combination of ammonium hydroxide (NH4OH) and urea, were used in addition to one allergen, Rhus (poison ivy). The model irritants and allergen were applied with an occlusive patch for 24 hr. Inflammation was scored with an expanded 10-point scale at 72 hr post application.
Most test protectants statistically suppressed SLS irritation and Rhus allergic reaction but not NH4OH- or urea-induced irritation. The researchers further utilized this model to evaluate putative skin-protective formulations. All formulations failed to inhibit NH4OH and urea irritation. Only paraffin wax in cetyl alcohol significantly reduced Rhus-allergic contact dermatitis (ACD). Three commercial formulationsa–c markedly suppressed SLS-ICD.
Tannic acid barrier protection: Shimizu and Maibach evaluated the barrier protectant tannic acid on human subjects utilizing squamometry. Either 5% tannic acid or distilled water (control) were applied to the forearms of subjects for 30 min; these pretreated sites were then dosed with 0.25%, 0.5% or 1% of SLS for 24 hr. Squamometric evaluation indicated that skin damage increased with SLS concentration in a dose-dependent manner, and tannic acid significantly reduced the damage.
Dimethicone/glycerin skin protectant: Patterson et al. determined the preventive effect of a skin protectant containing dimethicone and glycerin with various inactive ingredients in an aerosol foam against SLS-induced ICD and urushiol-induced ACD (poison ivy and poison oak). Skin reaction was assessed periodically for 10 days using a 0–7 point dermatitis scale. The formulation was significantly effective in reducing SLS irritation but did not prevent urushiol-ACD.
Hydrogel vs. petrolatum cream: Draelos conducted a randomized, double-blind, split-body study in a total of 80 men, women and children between the ages of newborn and 80 with the following dermatological conditions: household dermatitis, occupational hand dermatitis, latex glove-induced ICD, diaper dermatitis, cutaneous wounds and ACD. The subjects were given two identical jars—one containing a petrolatum-based cream and the other, a hydrogel-based barrier repair cream—and were instructed to apply one cream to half of their bodies and the other cream to the other half for four weeks. Both subject and investigator assessments were recorded by questionnaire. The hydrogel barrier/repair cream showed better skin improvement than the petrolatum-based cream in both subject assessment and investigator assessment.
Dimethicone ICD protection: Zhai et al. evaluated the efficacy of a dimethicone skin protectant lotion against SLS-induced ICD in humans with clinical visual grading and bioengineering techniques. Both forearms were pretreated either with the protectant test lotion or with its vehicle control prior to contact with SLS. After 30 min, 0.5% SLS was applied to each pretreated site for 24 hr. One additional site received SLS only. The protective effect of the dimethicone-lotion was determined by visual scoring (VS), TEWL measurement, skin color (a* value) and cutaneous blood flow volume (BFV). VS and TEWL data showed a significant decrease on the lotion-pretreated site in comparison with the SLS-treated site and the vehicle control site. However, BFV and a* values did not show a statistical difference between treated sites.
BC protection for hospital use: Berndt et al. investigated the efficacy of a BC and its vehicle in a field setting: two panels of 25 hospital nurses with mild signs of skin irritation were instructed to use one of the test products, BC or its vehicle, before contact with skin irritants over the course of four weeks. Effects of both preparations were studied weekly by clinical examination and bio- engineering measurements. Results showed no significant differences between BC and its vehicle. In both groups, clinical skin status improved and stratum corneum hydration increased significantly. Researchers concluded that the vehicle alone was capable of positively influencing skin status.
ROIT skin protective evaluation: Schnetz et al. utilized a short-time, repeated occlusive irritation test (ROIT) via a standardized protocol to evaluate skin protective products in two phases—i.e., 12 days and 5 days—in several clinical centers. Skin was treated by the irritants 0.5% SLS and toluene twice daily for 30 min. Inflammation was measured by bioengineering methods (TEWL and colorimetry) and clinical scoring. The 5-day protocol was sufficient to achieve significant results. Furthermore, in spite of the expected inter-center variations due to heterogeneity of the individual threshold of irritation, interpretation of clinical score and inter-instrumental variability, the ranking of the vehicles regarding reduction of the irritant reaction was consistent in all centers.
Oil-containing BC for health care use: McCormick et al. measured the efficacy of a BC and an oil-containing lotion for protecting the hands of health care workers with severe hand irritation. Objective and subjective parameters for scaling, cracking, weeping, bleeding and pain were blindly scored by two investigators weekly for four weeks. Subjects in both groups experienced marked improvement in overall hand condition, particularly in scaling, cracking and pain. Volunteers randomized to use the oil-containing lotion showed the greatest improvement.
LIBS evaluation of BCs: Sun et al. utilized laser-induced breakdown spectroscopy (LIBS) to evaluate the effect of BCs on human skin. Three representatives of commercial BCs promoted as being effective against lipophilic and hydrophilic substances were evaluated by measuring the zinc absorbed through the stratum corneum. Four consecutive SSBs were taken from the biceps of the forearms of six volunteers at 0.5 hr, 3 hr after BC application. The BCs provided appreciable protection against the penetration of both ZnCl2 and ZnO into the skin when compared with control skin (without BC treatment).
Aluminum chlorhydrate skin protection: Perrenoud et al. compared a BC formulated with 5% aluminum chlorhydrate as the active ingredient with its vehicle in 21 apprentice hairdressers, a double-blind crossover study. The efficacy of the creams was evaluated through clinical scores by researchers, biometric measurements and subjective opinions of the subjects. The researchers observed little difference in efficacy between the protective cream and the vehicle. However, aluminium chlorhydrate in the protective cream had a positive effect against work-related irritation.
ICD/ACD protection with o/w cream: De Paepe et al. investigated the effects of an o/w cream on barrier function in experimentally elicited ICD and ACD in 24 white female volunteers. In the ICD study, 1.25% SLS patches were applied to the forearms of volunteers, followed by twice daily application of the cream for 14 days. Researchers observed significantly improved TEWL in SLS-damaged skin, leading to a complete recovery on day 15, compared with the untreated site.
Preventing glove-induced ICD: Zhai et al. evaluated the ability of a model lipid emulsion to protect against glove-induced ICD. The test emulsion was applied to one hand while the opposite hand remained untreated. After 30 min, both hands were gloved for 3 hr. Skin conditions were evaluated by visual scoring, water sorption-desorption test, TEWL measurement and skin capacitance. This procedure was repeated for five days. Emulsion-treated hands showed significantly greater water holding capacity and lower TEWL values than untreated hands. Researchers concluded that the test emulsion minimized glove-induced ICD.
Dexpanthenol skin protection: Biro et al. investigated the efficacy of dexpanthenol to protect against irritation in a randomized, prospective, double-blind, placebo-controlled study. Twenty-five healthy volunteers were treated on the inner aspect of both forearms with a formulation containing 5% dexpanthenol or a placebo twice daily for 26 days. From days 15 to 22, 2% SLS was applied to these areas twice daily. Assessments included sebumetry, corneometry, pH value and clinical appearance (photographs). Researchers concluded that dexpanthenol exhibits protective effects against skin irritation.
Evaluating irritancy potential and efficacy: Diepgen et al. compared six commercially available skin care products with two standard experimental designs: the chamber scarification test to assess irritancy potential, and the ROIT, which was developed to evaluate the efficacy of skin care creams. Results showed that a high score in the chamber scarification test for skin irritation was not necessarily correlated to the products’ ability to impede SLS-induced irritant skin reactions. Three products exhibited low irritancy potential and were capable of reducing skin barrier damage induced by SLS while one product showed both irritant potential on scarified skin and also a modest capability to reduce skin irritation induced by SLS.
In vivo ROIT evaluation: Zur Muhlen et al. assessed the efficacy of three products through an in vivo ROIT method. The researchers demonstrated that a multiple emulsion provided the best protection against sodium dodecyl sulphate (SDS). They believed the multiple emulsion increased the content of skin lipids, which reduced the irritation induction or cell death caused by SDS.
Tape stripping protective evaluation: Teichmann et al. investigated the protective effectiveness of one commercially available BC, beeswax and white petrolatumd in six healthy volunteers. The researchers assessed the penetration behavior of Patent Blue V in water on the BC-pretreated skin, in comparison with an untreated site, by tape stripping. The commercial BC did not demonstrate a protective function (e.g., similar to the untreated site), while beeswax and white petrolatum were significant in their efficacy to protect barrier function.
Milk, cream and ointment protection: Ortonne and Queille-Roussel performed a single-center, blinded, randomized, controlled study in 20 healthy Caucasian women. During the irritation period, SLS at 5% was used to induce skin irritation on both forearms of each subject daily for five days. A milk (w/o emulsion), cream (w/o emulsion) and ointment (water-free emulsion)e were applied twice daily to three of the four test sites on days 1–5. The fourth site served as a control.
The cream was comprised of: water (aqua), caprylic/capric triglyceride, polyglyceryl-3 diisostearate, glycerin, dicaprylyl ether, cetearyl ethylhexanoate, petrolatum, Cera alba, sodium lactate, polyglyceryl-2 dipolyhydroxystearate, magnesium sulphate, lactic acid and ethylhexylglycerin. The milk contained: water (aqua), caprylic/capric triglyceride, cetearyl ethylhexanoate, urea, polyglyceryl-2 dipolyhydroxystearate, sodium lactate, glycerin, dicaprylyl ether, polyglyceryl-3 diisostearate, lactic acid, magnesium sulphate and ethylhexyl-glycerin. Finally, the ointment included: petrolatum, Paraffinum liquidum, microcrystalline wax, oleyl erucate, urea and Zea mays (corn) starch.
Visual readings, subjective symptom assessments, TEWL and colorimetric measurements, corneometry and skin microrelief macrophotographs were recorded on days 1–6. On day 6, TEWL with the cream or the ointment was found to be significantly lower than control. Skin capacitance was 94%, 100% and 85% of baseline value for the cream, milk and ointment, respectively, versus 72% for the control. All test products were well-tolerated and the researchers concluded that the products showed both protective properties against epidermal dysfunction and significant hydrating effects.
Pimecrolimus cream against irritation: Engel et al. tested the anti-inflammatory effect of pimecrolimus cream on SLS-induced skin damage in a randomized, placebo-controlled, observer-blinded study. SLS at 3% was applied under occlusion on the backs of 36 healthy volunteers for 24 hr. Subsequently, the test areas were treated for 24 hr on three consecutive days with pimecrolimus cream, 1% hydrocortisone in a hydrophilic ointment, and the vehicle alone. A control area remained untreated. The erythema index and the TEWL were measured. Pimecrolimus cream and 1% hydrocortisone cream significantly reduced the SLS-induced erythema but did not have a significant effect on TEWL.
Conclusion
The studies described in this literature review evaluate the efficacy of anti-irritant agents for reducing ICD in human skin. For instance, tannic acid significantly reduced damage, and beeswax and white petrolatum were significant in their efficacy to protect barrier function. As noted, this investigation of anti-irritants will continue in the April 2011 edition with the efficacy of moisturizers and anti-irritant substances, as well as provide an overall interpretation.
Reproduction of the article without expressed consent is strictly prohibited.
References
Send e-mail to [email protected].
1. C Ford and HI Maibach, Anti-irritants: Myth or reality? Overview, ch 83 in Dermatotoxicology, 7th edn, H Zhai, Wilhelm and HI Maibach, eds, CRC Press, Boca Raton, FL (2008) pp 743–748
2. W Wigger-Alberti and P Elsner, Contact dermatitis due to irritation, ch 11 in Handbook of Occupational Dermatol, L Kanerva, P Elsner, JE Wahlberg and HI Maibach, eds, Springer, Berlin (2000) pp 99–110
3. S Weltfriend and HI Maibach, Irritant dermatitis: Clinical heterogeneity and contributing factors, ch 13 in Dermatotoxicology, 7th edn, H Zhai, KP Wilhelm and HI Maibach, eds, CRC Press, Boca Raton, FL (2008) pp 125–138
4. H Zhai and HI Maibach, Moisturizers in preventing irritant contact dermatitis: An overview, Contact Dermatitis 38 241–244 (1998)
5. H Zhai and HI Maibach, Barrier creams and emollients, in Irritant Dermatitis, AL Chew and HI Maibach, eds, Springer, Berlin (2006) pp 479–485
6. M Yokota and HI Maibach, Moisturizer effect on irritant dermatitis: An overview, Contact Dermatitis 55 65–72 (2006)
7. H Zhai and HI Maibach, Protection from irritants, Current Problems in Dermatology 34 47–57 (2007)
8. H Zhai and HI Maibach, Barrier creams, ch 32 in Dermatotoxicology, 7th edn, H Zhai, KP Wilhelm, and HI Maibach, eds, CRC Press, Boca Raton, FL (2008) pp 299–302
9. H Zhai, A Anigbogu and Maibach HI, Irritant and allergic contact dermatitis treatment, ch 76 in Dermatotoxicology, 7th edn, H Zhai, KP Wilhelm and HI Maibach, eds, CRC Press, Boca Raton, FL (2008) pp 689–695
10. M Lodén, Moisturizers, in Cosmeceuticals. Drugs vs. Cosmetics, P Elsner and HI Maibach, eds, Marcel Dekker, New York (2000) pp 73–96
11. JJ Leyden and AV Rawlings, eds, Skin Moisturization, Marcel Dekker, New York (2002)
12. H-Y Thong, J Spoo, P Elsner, P Kleesz, and HI Maibach, Occupational skin protection– A plea for definitions and a better understanding, Dermatologie in Beruf und Umwelt 56 3–6 (2008)
13. T-F Tsai, Water: Is it an irritant, ch 29 in Dermatotoxicology, 7th edn, H Zhai, KP Wilhelm and HI Maibach, eds, CRC Press, Boca Raton, FL (2008) pp 279–282